作为潜在多靶点抗抑郁剂的芳基丙胺衍生物的设计、合成和生物学评价。
IF 3
3区 医学
Q2 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
本研究设计、合成并评估了一系列新型芳基丙胺衍生物,将其作为潜在的多靶点抗抑郁剂。其中,化合物(R)-13j显示出独特的药理学特征,对5-羟色胺和去甲肾上腺素转运体(SERT/NET)具有极佳的抑制效力,对5-HT2A/2C受体具有高亲和力,对组胺H1、肾上腺素能α1受体和hERG通道(减少QT间期延长)具有低亲和力。分子对接研究提供了(R)-13j 与 SERT 和 5-HT2A/2C 受体复合物的合理结合模型。在动物模型中,化合物(R)-13j能剂量依赖性地减少小鼠在尾悬试验(TST)和强迫游泳试验(FST)中的不动时间,与度洛西汀相比疗效更高,且对运动活动无刺激作用。此外,(R)-13j 复合物还能显著缩短 ACTH 诱导的大鼠耐药抑郁症(TRD)模型的静止时间。此外,(R)-13j 复合物的急性毒性阈值也高于度洛西汀。此外,(R)-13j 复合物在小鼠体内具有良好的药代动力学特征。综上所述,化合物(R)-13j可能是治疗抑郁症的一类新型药物。本文章由计算机程序翻译,如有差异,请以英文原文为准。
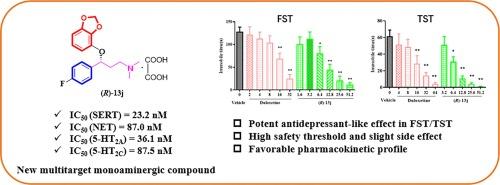
Design, synthesis and biological evaluation of arylpropylamine derivatives as potential multi-target antidepressants
In this study, a series of novel arylpropylamine derivatives were designed, synthesized and evaluated as potential multi-target antidepressants. Among them, compound (R)-13j displayed unique pharmacological features, exhibiting excellent inhibitory potency against serotonin and noradrenaline transporters (SERT/NET) and high affinity for 5-HT2A/2C receptor, and showing low affinity for histamine H1, adrenergic α1 receptors and hERG channels (to reduce QT interval prolongation). Molecular docking studies provided a rational binding model of (R)-13j in complex with SERT and 5-HT2A/2C receptor. In animal models, compound (R)-13j dose-dependently reduced the immobility time in the tail suspension test (TST) and the forced swimming test (FST) in mice, with higher efficacy when compared to duloxetine, and showed no stimulatory effect on the locomotor activity. Moreover, compound (R)-13j significantly shortened the immobility time in the ACTH-induced rat model of treatment-resistant depression (TRD). Furthermore, compound (R)-13j also exhibited a higher threshold for acute toxicity than duloxetine. In addition, compound (R)-13j possessed a favorable pharmacokinetic profile in mice. Taken together, compound (R)-13j may constitute a novel class of drugs for the treatment of depression.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Bioorganic & Medicinal Chemistry
医学-生化与分子生物学
CiteScore
6.80
自引率
2.90%
发文量
413
审稿时长
17 days
期刊介绍:
Bioorganic & Medicinal Chemistry provides an international forum for the publication of full original research papers and critical reviews on molecular interactions in key biological targets such as receptors, channels, enzymes, nucleotides, lipids and saccharides.
The aim of the journal is to promote a better understanding at the molecular level of life processes, and living organisms, as well as the interaction of these with chemical agents. A special feature will be that colour illustrations will be reproduced at no charge to the author, provided that the Editor agrees that colour is essential to the information content of the illustration in question.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


