灵芝 Meroterpenoids 的一般入口:Applanatumol E、H 和 I、Lingzhilactone B、Meroapplanin B 和 Lingzhiol 的合成。
IF 4.9
1区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
摘要
灵芝美拉普兰素是一种从真菌中提取的混合天然产物,含有一个 1,2,4-三取代苯环和一个多环萜类化合物部分。其代表产品 applanatumol E、H 和 I、lingzhilactone B 以及 meroapplanin B 都具有与炔连接的相同双环内酯分子。利用光-弗里斯重排作为关键步骤,可以普遍进入这些天然产物的合成。在合成灵芝醇的四环框架时,我们采用了强大的光氧化氧化脱羧/弗里德尔-卡夫顺序。本文章由计算机程序翻译,如有差异,请以英文原文为准。
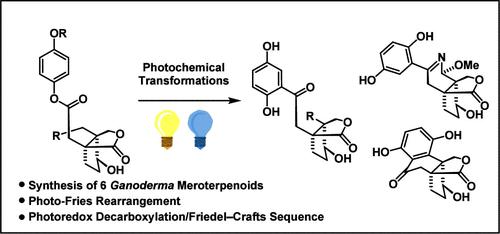
A General Entry to Ganoderma Meroterpenoids: Synthesis of Applanatumol E, H, and I, Lingzhilactone B, Meroapplanin B, and Lingzhiol.
Ganoderma meroterpenoids are fungal derived hybrid natural product class containing a 1,2,4-trisubstituted benzene ring and a polycyclic terpenoid part. The representatives applanatumol E, H and I, lingzhilactone B, and meroapplanin B share the same bicyclic lactone moiety connected to the arene. Employing photo-Fries rearrangements as the key step enabled a general entry to these natural products. For the synthesis of the tetracyclic framework of lingzhiol, we made use of a powerful photoredox oxidative decarboxylation/Friedel-Crafts sequence.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Organic Letters
化学-有机化学
CiteScore
9.30
自引率
11.50%
发文量
1607
审稿时长
1.5 months
期刊介绍:
Organic Letters invites original reports of fundamental research in all branches of the theory and practice of organic, physical organic, organometallic,medicinal, and bioorganic chemistry. Organic Letters provides rapid disclosure of the key elements of significant studies that are of interest to a large portion of the organic community. In selecting manuscripts for publication, the Editors place emphasis on the originality, quality and wide interest of the work. Authors should provide enough background information to place the new disclosure in context and to justify the rapid publication format. Back-to-back Letters will be considered. Full details should be reserved for an Article, which should appear in due course.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


