从 Platycladi cacumen 中提取的桧黄酮是肠道微生物 Loop-1 β-葡萄糖醛酸酶的强效广谱抑制剂:抑制动力学和分子模拟。
IF 5.4
2区 医学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
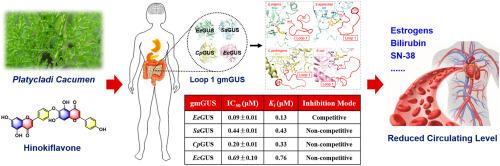
肠道微生物Loop-1 β-葡萄糖醛酸酶(gmGUS)通过肠肝再循环调节伊立替康活性代谢产物SN38的水平,在伊立替康诱导的胃肠道毒性中发挥着重要作用。本研究旨在探讨 Platycladi cacumen 及其主要成分 hinokiflavone 对四种不同类型的 Loop-1 gmGUS(EeGUS、SaGUS、CpGUS 和 EcGUS)的抑制作用和机制。结果表明,Platycladi cacumen乙醇提取物对四种gmGUS均有较强的广谱抑制作用,其中桧黄酮对EeGUS、SaGUS、CpGUS和EcGUS均有较强的抑制作用,IC50值分别为0.09 ± 0.01 μM、0.44 ± 0.01 μM、0.20 ± 0.01 μM和0.69 ± 0.10 μM。抑制动力学分析表明,桧黄酮是EeGUS的强竞争性抑制剂,Ki值为0.13 μM,而它对SaGUS、CpGUS和EcGUS的抑制作用是非竞争性的,Ki值分别为0.43 μM、0.33 μM和0.76 μM。对接模拟显示,桧黄酮能与EeGUS催化位点上的Tyr-485和Glu-516紧密结合,还能与SaGUS(Asn-362)、CpGUS(Phe-363、Met-364、Ala-365和Arg-375)和EcGUS(Leu-361)环结构中的氨基酸产生强相互作用,干扰底物进入催化口袋。总之,这些结果证实 Platycladi cacumen 中的桧黄酮是一种天然存在的强效 gmGUS 抑制剂,具有广泛的效率,表明桧黄酮将有助于减轻伊立替康治疗中的肠道毒性。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Hinokiflavone from Platycladi cacumen as a potent broad-spectrum inhibitor of gut microbial Loop-1 β-glucuronidases: Inhibition kinetics and molecular simulation
Gut microbial Loop-1 β-glucuronidases (gmGUS) played an important role in irinotecan-induced gastrointestinal toxicity by regulating the level of its active metabolite SN38 through enterohepatic recirculation. gmGUS inhibition has emerged as a promising approach to relieve its dose-limiting intestinal toxicity and improve its medication efficacy. This study aims to investigate the inhibitory effects and mechanisms of Platycladi cacumen and its main constituent hinokiflavone against four different types of Loop-1 gmGUS (EeGUS, SaGUS, CpGUS and EcGUS). Our results showed that the ethanol extract of Platycladi cacumen displayed strong broad-spectrum inhibition against four gmGUS, and hinokiflavone could potently inhibit EeGUS, SaGUS, CpGUS and EcGUS with IC50 values of 0.09 ± 0.01 μM, 0.44 ± 0.01 μM, 0.20 ± 0.01 μM and 0.69 ± 0.10 μM, respectively. Inhibition kinetic analyses demonstrated that hinokiflavone acted as a strong competitive inhibitor of EeGUS with Ki value of 0.13 μM, while it displayed non-competitive inhibition against SaGUS, CpGUS and EcGUS, with the Ki values of 0.43 μM, 0.33 μM and 0.76 μM, respectively. Docking simulations revealed that hinokiflavone could tightly bind with Tyr-485 and Glu-516 in catalytic sites of EeGUS, as well it created strong interactions with amino acids in loop structures of SaGUS (Asn-362), CpGUS (Phe-363, Met-364, Ala-365 and Arg-375) and EcGUS (Leu-361) to interfere the substrate entry into the catalytic pocket. Collectively, these results confirmed that hinokiflavone from Platycladi cacumen is a potent naturally occurring inhibitor of gmGUS with broad efficiency, suggesting hinokiflavone will be helpful for alleviating intestinal toxicity in irinotecan therapy.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
7.70
自引率
3.90%
发文量
410
审稿时长
36 days
期刊介绍:
Chemico-Biological Interactions publishes research reports and review articles that examine the molecular, cellular, and/or biochemical basis of toxicologically relevant outcomes. Special emphasis is placed on toxicological mechanisms associated with interactions between chemicals and biological systems. Outcomes may include all traditional endpoints caused by synthetic or naturally occurring chemicals, both in vivo and in vitro. Endpoints of interest include, but are not limited to carcinogenesis, mutagenesis, respiratory toxicology, neurotoxicology, reproductive and developmental toxicology, and immunotoxicology.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


