FUNDC1 介导的有丝分裂调节光损伤,与 PINK1/Parkin 依赖性途径无关。
IF 7.1
2区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
背景:紫外线B(UVB)通过有丝分裂引发一种促生存反应,但FUNDC1介导的有丝分裂在光损伤皮肤中的作用仍未得到探索:明确有丝分裂在 UVB 诱导的光损伤皮肤中的功能:为了研究 FUNDC1 介导的有丝分裂在 UVB 诱导的线粒体损伤和细胞凋亡中的作用,使用腺相关病毒在 C57BL/6 小鼠中敲除 FUNDC1。此外,还使用慢病毒在 HaCaT 细胞中进行了 FUNDC1 的过表达和敲除。对一组暴露于阳光的人类皮肤样本和对照样本进行了综合分析,以评估 FUNDC1 的表达水平:结果:紫外线诱导的 C57BL/6 小鼠背侧皮肤出现光损伤,包括红斑、脱屑、糜烂和结痂。PINK1、Parkin和BNIP3的表达水平没有发生显著变化,而FUNDC1的表达与LC3B一起持续下降。细胞色素 C、Bax 和裂解-caspase3 表达上调,而 Bcl2 表达下调。UVB 诱导的 HaCaT 细胞出现线粒体损伤,伴随着 FUNDC1 的下调和 BNIP3 的上调,而 PINK1 和 Parkin 则无明显变化。FUNDC1 过表达导致 mtROS 增加,线粒体膜电位和 ATP 水平下降,表明线粒体完全清除,细胞死亡加剧。敲除 FUNDC1 可保护小鼠免受 UVB 引起的光损伤,并通过激活 PINK1/Parkin 依赖性有丝分裂的代偿性,减轻 HaCaT 细胞的线粒体损伤和细胞凋亡,PINK1 和 Bcl2 的上调以及 Bax 的下调证明了这一点。与未暴露于阳光下的对照组相比,暴露于阳光下的人体皮肤样本中 FUNDC1+细胞的数量有所减少:结论:FUNDC1介导的有丝分裂调节皮肤光损伤,并提供了一种抵抗光损伤的新机制,是未来治疗干预的潜在靶点。本文章由计算机程序翻译,如有差异,请以英文原文为准。
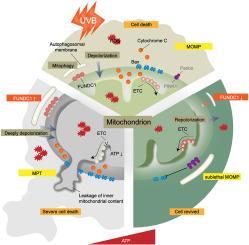
FUNDC1-mediated mitophagy regulates photodamage independently of the PINK1/Parkin-dependent pathway
Background
Ultraviolet B(UVB) triggers a pro-survival response through mitophagy, but the role of FUNDC1-mediated mitophagy in photodamaged skin remains unexplored.
Objectives
To clarify the function of mitophagy in UVB-induced photodamaged skin.
Methods
To investigate the role of FUNDC1-mediated mitophagy in UVB-induced mitochondrial damage and cell apoptosis, FUNDC1 knockdown in C57BL/6 mice was performed using adeno-associated virus. Additionally, FUNDC1 overexpression and knockdown in HaCaT cells were conducted using lentivirus. A comprehensive analysis was conducted on a panel of human sun-exposed skin samples, alongside control samples, to assess the expression levels of FUNDC1.
Results
In UVB-induced C57BL/6 mice, the dorsal skin showed photodamage including erythema, scaling, erosion, and scabs. The expression levels of PINK1, Parkin, and BNIP3 did not show significant changes, while FUNDC1 expression consistently declined along with LC3B. Cytochrome C, Bax, and cleaved-caspase3 were upregulated, while Bcl2 was downregulated. UVB-induced HaCaT cells showed mitochondrial damage, accompanied by FUNDC1 downregulation and BNIP3 upregulation, while PINK1 and Parkin showed no significant changes. FUNDC1 overexpression led to an increase in mtROS and a decrease in mitochondrial membrane potential and ATP levels, indicating complete mitochondrial clearance and exacerbated cell death. FUNDC1 knockdown protected against UVB-induced photodamage in mice and mitigated mitochondrial damage and apoptosis in HaCaT cells by activating compensatory PINK1/Parkin-dependent mitophagy, which was evidenced by upregulation of PINK1 and Bcl2 and downregulation of Bax. In human sun-exposed skin samples, there was a decrease in the number of FUNDC1+ cells compared with non-sun-exposed controls.
Conclusions
FUNDC1-mediated mitophagy regulates skin photodamage and provides a novel mechanism for resisting photodamage, presenting a potential target for future therapeutic interventions.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Free Radical Biology and Medicine
医学-内分泌学与代谢
CiteScore
14.00
自引率
4.10%
发文量
850
审稿时长
22 days
期刊介绍:
Free Radical Biology and Medicine is a leading journal in the field of redox biology, which is the study of the role of reactive oxygen species (ROS) and other oxidizing agents in biological systems. The journal serves as a premier forum for publishing innovative and groundbreaking research that explores the redox biology of health and disease, covering a wide range of topics and disciplines. Free Radical Biology and Medicine also commissions Special Issues that highlight recent advances in both basic and clinical research, with a particular emphasis on the mechanisms underlying altered metabolism and redox signaling. These Special Issues aim to provide a focused platform for the latest research in the field, fostering collaboration and knowledge exchange among researchers and clinicians.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


