多粘菌素和八肽对人类肥大细胞的不同 MRGPRX2 依赖性激活作用
IF 4.2
3区 医学
Q1 PHARMACOLOGY & PHARMACY
引用次数: 0
摘要
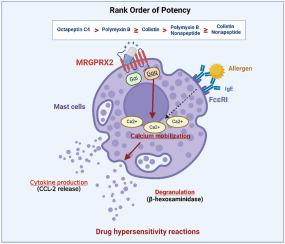
多重耐药革兰氏阴性菌的出现使人们对多粘菌素和新型多粘菌素类似物(如壬肽和八肽)的抗菌活性重新产生了兴趣。在某些人体内,临床使用的多粘菌素可通过激活肥大细胞引起急性超敏反应,最近的一项研究将这种效应归因于激活 MAS 相关 G 蛋白偶联受体 X2(MRGPRX2)。在本研究中,我们使用了表达人类 MRGPRX2 的 HEK293 细胞和人类肥大细胞系 LAD2 来鉴定更广泛的多粘菌素家族的活性。八肽 C4、多粘菌素 B 和大肠杆菌素能在 LAD2 肥大细胞中产生浓度依赖性的钙动员、脱颗粒和 CCL-2(MCP-1)释放,其中前者的效力很强。CRISPR-Cas9 敲除 LAD2 细胞中的 MRGPRX2 和新的 MRGPRX2 反激动剂可显著降低钙动员、脱颗粒和 CCL-2 释放,这表明 MRGPRX2 的表达具有依赖性。相比之下,多粘菌素非肽的钙动员作用要弱得多,而且不能诱导 LAD2 细胞的功能性脱颗粒。我们的研究结果证实,多粘菌素相关抗生素诱导的肥大细胞活化是依赖于 MRGPRX2 的,并揭示了八肽 C4 可能更容易在临床上引发即刻超敏反应,而非肽类则不那么容易。更好地了解多粘菌素相关抗生素之间 MRGPRX2 激活差异的机制非常重要,因为这有助于设计更安全的新型多粘菌素,并指导现有多粘菌素药物的最佳临床使用。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Differential MRGPRX2-dependent activation of human mast cells by polymyxins and octapeptins
The emergence of multi-drug resistant Gram-negative bacteria has led to renewed interest in the antimicrobial activity of polymyxins and novel polymyxin analogues (e.g. nonapeptides and octapeptin). In some individuals, clinically used polymyxins can cause acute hypersensitivity reactions through mast cell activation, with a recent study attributing this effect to activation of the MAS-related G protein-coupled receptor X2 (MRGPRX2). In the present study, HEK293 cells expressing human MRGPRX2 and the human mast cell line LAD2 were used to characterize the activity of the broader family of polymyxins. Octapeptin C4, polymyxin B and colistin produced concentration-dependent calcium mobilization, degranulation, and CCL-2 (MCP-1) release in LAD2 mast cells, with the former being highly potent. CRISPR-Cas9 knockdown of MRGPRX2 in LAD2 cells and a MRGPRX2 inverse agonist caused a significant reduction in calcium mobilization, degranulation, and CCL-2 release, demonstrating dependency on MRGPRX2 expression. In contrast, polymyxin nonapeptides were far less potent calcium mobilisers and failed to induce functional degranulation in LAD2 cells. Our results confirm that activation of mast cells induced by polymyxin-related antibiotics is MRGPRX2-dependent and reveal that octapeptin C4 might be more liable, whilst nonapeptides are less liable, to trigger immediate hypersensitivity reactions clinically. The mechanism underpinning the difference in MRGPRX2 activation between polymyxin-related antibiotics is important to better understand as it may help design new, safer polymyxins and guide the optimal clinical use of existing polymyxin drugs.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
9.00
自引率
0.00%
发文量
572
审稿时长
34 days
期刊介绍:
The European Journal of Pharmacology publishes research papers covering all aspects of experimental pharmacology with focus on the mechanism of action of structurally identified compounds affecting biological systems.
The scope includes:
Behavioural pharmacology
Neuropharmacology and analgesia
Cardiovascular pharmacology
Pulmonary, gastrointestinal and urogenital pharmacology
Endocrine pharmacology
Immunopharmacology and inflammation
Molecular and cellular pharmacology
Regenerative pharmacology
Biologicals and biotherapeutics
Translational pharmacology
Nutriceutical pharmacology.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


