高密度培养诱导的静止癌细胞显示出胆固醇介导的存活和肺转移特性。
IF 6.4
1区 医学
Q1 ONCOLOGY
引用次数: 0
摘要
背景:转移过程是一个多方面的、高度侵袭性的过程,是导致死亡的主要原因。转移过程中静止癌细胞在循环系统中的存活至关重要,但由于缺乏公认的静止癌细胞模型,我们的理解能力受到限制:方法:我们利用高密度培养技术开发了一种静止癌细胞模型。方法:我们利用高密度培养技术建立了一个静止癌细胞模型,并基于 scRNA-seq 分析、IP-MS、代谢组学、小鼠肺转移模型、胆固醇测定、PLA 及其他分子实验,探索其分子机制。利用免疫荧光、原子力显微镜、FluidFM和剪切应力刺激等手段分析了细胞骨架和膜特性对机械力抵抗的贡献:结果:我们建立了一个由高密度培养诱导的静止癌细胞模型。单细胞 RNA 测序(scRNA-seq)分析表明,CDC25A 在向静止过渡的过程中起着关键作用,其表达量在静止状态下显著升高。消耗 CDC25A 会导致增殖能力增强,并减少高密度条件下的转移。从机理上讲,静止细胞中上调的 CDC25A 会通过内质体途径加强胆固醇代谢,导致细胞周期停滞。胆固醇的增加强化了细胞骨架,改变了膜特性,提高了循环系统对机械力的抵抗力:结论:CDC25A通过内质体途径明显增加了静止期癌细胞的胆固醇代谢,导致细胞骨架和膜特性发生显著变化,从而增强了循环系统对机械力的抵抗力,促进了肺转移。在高密度培养中,静止期癌细胞通过内质体途径上调CDC25A的胆固醇代谢,从而增强循环系统对机械力的抵抗力,促进肺转移。本文章由计算机程序翻译,如有差异,请以英文原文为准。
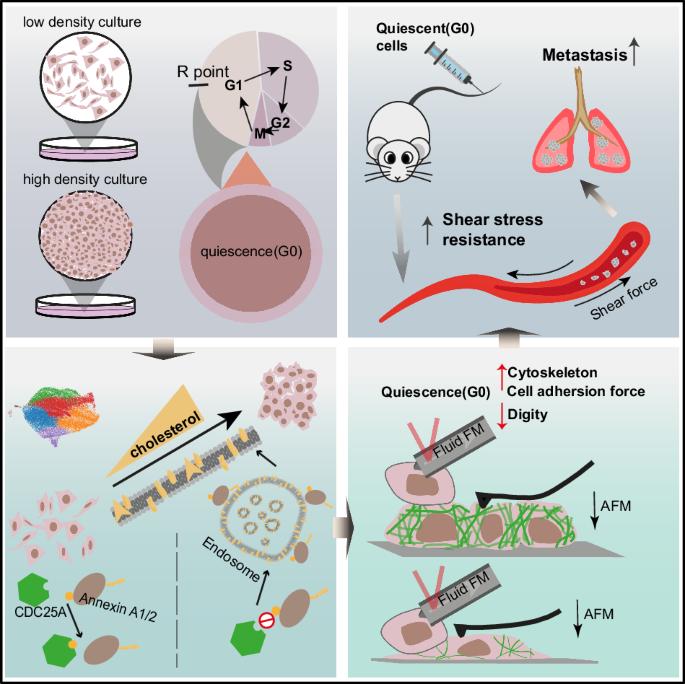
Quiescent cancer cells induced by high-density cultivation reveals cholesterol-mediated survival and lung metastatic traits
The metastatic cascade, a multifaceted and highly aggressive process, is the primary cause of mortality. The survival of quiescent cancer cells in circulatory system during metastasis is crucial, yet our comprehension is constrained by the absence of universally accepted quiescent cancer models. We developed a quiescent cancer cell model using high-density cultivation. Based on the scRNA-seq analysis, IP-MS, metabolomics, mouse lung metastasis models, cholesterol assay, PLA and other molecular experiments, we explored the molecular mechanism. Immunofluorescence, atomic force microscope, FluidFM, and shear stress stimulation were used to analyze the cytoskeleton and membrane properties contributing to mechanical force resistance. We established a quiescent cancer cell model induced by high-density cultivation. Single-cell RNA sequencing (scRNA-seq) analysis reveals that CDC25A plays a crucial role in the transition to quiescence, with its expression significantly elevated in the quiescent state. Depletion of CDC25A leads to an increased proliferative capacity, and reduced metastasis under high-density conditions. Mechanistically, upregulated CDC25A in quiescent cells enhances cholesterol metabolism via endosome pathways, leading to cell cycle arrest. This increase in cholesterol reinforces the cytoskeleton, alters membrane properties, and improves resistance to mechanical forces in circulatory system. CDC25A significantly increased the cholesterol metabolism through endosome pathway in quiescent cancer cells, leading to the significant changes in cytoskeleton and membrane properties so as to enhance the resistance of mechanical force in circulatory system, facilitating lung metastasis.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

British Journal of Cancer
医学-肿瘤学
CiteScore
15.10
自引率
1.10%
发文量
383
审稿时长
6 months
期刊介绍:
The British Journal of Cancer is one of the most-cited general cancer journals, publishing significant advances in translational and clinical cancer research.It also publishes high-quality reviews and thought-provoking comment on all aspects of cancer prevention,diagnosis and treatment.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


