活化的 CD8+ T 细胞上的 SLAMF7 (CD319) 通过环境线索启动细胞毒性效应细胞反应
IF 13.7
1区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
CD8+ T 细胞反应是由细胞间受体与配体相互作用调节的精心安排的过程。这些相互作用关键性地控制着 CD8+ T 细胞群的动态,这对克服病毒感染或癌症等威胁至关重要。然而,这些动态机制仍未完全阐明。在这里,我们发现了 CD8+ T 细胞上的自锁表面受体 SLAMF7(CD319)在细胞毒性 T 细胞反应启动过程中的一种迄今未知的 T 细胞转介功能。根据其在 T 效应细胞上与细胞毒性相关的表达,我们发现 CD8+ T 细胞可利用 SLAMF7 将环境线索转化为细胞相互作用和信息交换。事实上,SLAMF7 可促进形成剂量依赖性的稳定同型接触,最终形成稳定的细胞接触、法定人数群以及扩增和分化承诺。通过牵引试验和网络分析,我们发现了新型 SLAMF7 结合的细胞内信号分子,包括 CRK、CRKL 和 Nck 适配体,它们参与了 T 细胞接触的形成,并可能介导 SLAMF7 在传感和粘附方面的功能。因此,在 CD8+ T 细胞识别抗原的过程中提供 SLAMF7 信号会增强它们的整体数量,尤其是对低亲和力抗原的反应,从而显著提高它们的增殖和细胞毒性能力。总之,我们发现并鉴定了细胞毒性 T 淋巴细胞反应程序的强效启动因子,揭示了改善 CD8+ T 细胞对弱病毒或肿瘤相关抗原反应决策的先进机制,从而加强了我们对此类对手的防御能力。本文章由计算机程序翻译,如有差异,请以英文原文为准。

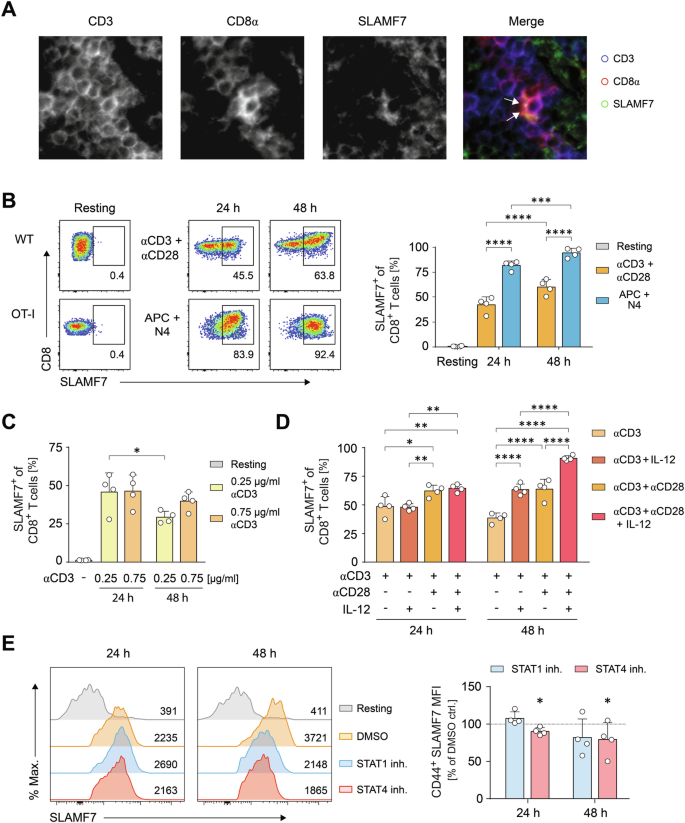
SLAMF7 (CD319) on activated CD8+ T cells transduces environmental cues to initiate cytotoxic effector cell responses
CD8+ T-cell responses are meticulously orchestrated processes regulated by intercellular receptor:ligand interactions. These interactions critically control the dynamics of CD8+ T-cell populations that is crucial to overcome threats such as viral infections or cancer. Yet, the mechanisms governing these dynamics remain incompletely elucidated. Here, we identified a hitherto unknown T-cell referred function of the self-ligating surface receptor SLAMF7 (CD319) on CD8+ T cells during initiation of cytotoxic T-cell responses. According to its cytotoxicity related expression on T effector cells, we found that CD8+ T cells could utilize SLAMF7 to transduce environmental cues into cellular interactions and information exchange. Indeed, SLAMF7 facilitated a dose-dependent formation of stable homotypic contacts that ultimately resulted in stable cell-contacts, quorum populations and commitment to expansion and differentiation. Using pull-down assays and network analyses, we identified novel SLAMF7-binding intracellular signaling molecules including the CRK, CRKL, and Nck adaptors, which are involved in T-cell contact formation and may mediate SLAMF7 functions in sensing and adhesion. Hence, providing SLAMF7 signals during antigen recognition of CD8+ T cells enhanced their overall magnitude, particularly in responses towards low-affinity antigens, resulting in a significant boost in their proliferation and cytotoxic capacity. Overall, we have identified and characterized a potent initiator of the cytotoxic T lymphocyte response program and revealed advanced mechanisms to improve CD8+ T-cell response decisions against weak viral or tumor-associated antigens, thereby strengthening our defense against such adversaries.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Cell Death and Differentiation
生物-生化与分子生物学
CiteScore
24.70
自引率
1.60%
发文量
181
审稿时长
3 months
期刊介绍:
Mission, vision and values of Cell Death & Differentiation:
To devote itself to scientific excellence in the field of cell biology, molecular biology, and biochemistry of cell death and disease.
To provide a unified forum for scientists and clinical researchers
It is committed to the rapid publication of high quality original papers relating to these subjects, together with topical, usually solicited, reviews, meeting reports, editorial correspondence and occasional commentaries on controversial and scientifically informative issues.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


