与 TNF 抑制剂相比,JAK 抑制剂或托珠单抗类药物对类风湿性关节炎患者心血管的安全性:随机临床试验的系统综述及传统和贝叶斯网络荟萃分析。
IF 4.6
2区 医学
Q1 RHEUMATOLOGY
引用次数: 0
摘要
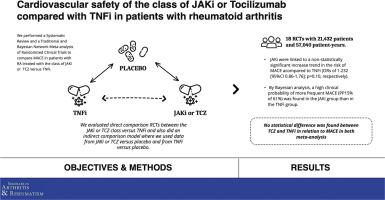
背景:我们旨在比较类风湿关节炎(RA)患者接受JAK抑制剂(JAKi)或妥西珠单抗(TCZ)治疗与肿瘤坏死因子(TNF)抑制剂(TNFi)治疗的主要不良心血管事件(MACE)和全因死亡(ACD)风险:我们对截至2024年5月的6个医学数据库进行了系统性回顾,以了解2-4期随机对照试验(RCT)的情况,这些试验评估了接受TCZ或JAKi(干预组)治疗的RA患者与对照组(TNFi或安慰剂)的对比情况。研究数据由3名研究人员独立评估。偏倚风险采用 Cochrane 协作工具进行评估。我们采用随机效应进行了网络荟萃分析,以评估与 TNFi 相比发生 MACE(主要结局)和 ACD(次要结局)的风险。我们还根据贝叶斯定理计算了主要和次要结局增加15%或更多的后验概率(PP15%):这项荟萃分析包括18项RCT,共21432名患者,57040个患者年。与 TNFi 相比,JAKi 与 MACE 和 ACD 风险的增加无统计学意义(ORs 分别为 1.232[95%CI 0.86-1.76];P = 0.56 和 ORs = 1.3903[95%CI 0.94-2.07];P = 0.10)。通过贝叶斯分析,发现JAKi组比TNFi组临床上发生MACE(PP15%,61%)和ACD(PP15%,84%)的概率更高。在传统荟萃分析中,TCZ和TNFi在MACE(1.029 [95%CI 0.75 -1.40]; p = 0.86)和ACD(1.072 [95%CI 0.78-1.48]; p = 0.67])方面没有统计学差异。在贝叶斯方法中,临床相关性差异的概率较低(MACE的PP15%为11%,ACD的PP15%为25%):本研究的主要局限性在于使用托法替尼以外的 JAKi 发生的事件较少,这反映了口服检查的重要性。尽管如此,这些数据还是加强了监管机构和风湿病学会关于在RA情况下使用JAKi的建议,但最重要的是,由于现有的结果数据仍然较少且异质性较强,因此需要进行更多涉及JAKi主要安全性结果的直接比较研究。在两项荟萃分析中,均未发现TNFi与TCZ之间存在差异。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Cardiovascular safety of the class of JAK inhibitors or tocilizumab compared with TNF inhibitors in patients with rheumatoid arthritis: Systematic review and a traditional and Bayesian network meta-analysis of randomized clinical trials
Background
We aimed to compare the risk of major adverse cardiovascular events (MACE) and all-cause of death (ACD) in patients with rheumatoid arthritis (RA) treated with JAK inhibitors (JAKi) or tocilizumab (TCZ) versus tumor necrosis factor (TNF) inhibitors (TNFi).
Methods
We performed a systematic review of six medical databases until May, 2024 for phase 2–4 randomized controlled trials (RCTs) evaluating patients with RA treated with TCZ or JAKi (intervention arm) compared with controls (TNFi or placebo). The study data were independently assessed by 3 investigators. The risk of bias was assessed using the Cochrane Collaboration tool. We performed a network meta-analysis with random effects to evaluate the risk of MACE (primary outcome) and ACD (secondary outcome) compared to TNFi. We also calculated the posterior probability of increasing the primary and secondary outcomes by 15% or more (PP15%) following Bayes' theorem.
Results
This meta-analysis included 18 RCTs with 21,432 patients and 57,040 patient-years. JAKi were linked to a non-statistically significant increase in the risk of MACE and ACD as compared to TNFi (ORs of 1.232 [95%CI 0.86–1.76]; p = 0.56 and ORs = 1.3903[95%CI 0.94–2.07]; p = 0.10, respectively). By Bayesian analysis, a high clinical probability of more frequent MACE (PP15% of 61%) and ACD (PP15% of 84%) was found in the JAKi group than in the TNFi group. No statistical difference was found between TCZ and TNFi in relation to MACE (1.029 [95%CI 0.75 -1.40]; p = 0.86) and ACD (1.072 [95%CI 0.78–1.48]; p = 0.67]) in the traditional meta-analysis. In the Bayesian approach, the probability of a difference in clinical relevance was low (PP15% for MACE of 11% and PP15% for ACD of 25%).
Discussion
The main limitation of this study is the small number of events with JAKi other than tofacitinib, reflecting the importance of ORAL SURVEILLANCE. Despite this, these data reinforce the recommendations of regulatory agencies and rheumatology societies on the use of JAKi in the context of RA, but above all call for more direct comparison studies involving primary safety outcomes with JAKi, since the outcome data available are still small and heterogeneous. In both meta-analyses, no difference was found between TNFi and TCZ.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
9.20
自引率
4.00%
发文量
176
审稿时长
46 days
期刊介绍:
Seminars in Arthritis and Rheumatism provides access to the highest-quality clinical, therapeutic and translational research about arthritis, rheumatology and musculoskeletal disorders that affect the joints and connective tissue. Each bimonthly issue includes articles giving you the latest diagnostic criteria, consensus statements, systematic reviews and meta-analyses as well as clinical and translational research studies. Read this journal for the latest groundbreaking research and to gain insights from scientists and clinicians on the management and treatment of musculoskeletal and autoimmune rheumatologic diseases. The journal is of interest to rheumatologists, orthopedic surgeons, internal medicine physicians, immunologists and specialists in bone and mineral metabolism.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


