通过激活 FGF21 限制蛋氨酸摄入可减轻糖尿病相关的认知障碍。
IF 11.9
1区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
葡萄糖代谢紊乱可能导致糖尿病相关认知能力下降(DACI)。蛋氨酸限制(MR)饮食已成为管理葡萄糖稳态的一种潜在饮食策略。然而,MR 对 DACI 的影响及其内在机制尚未完全阐明。在这里,我们发现从 8 周龄开始进行为期 13 周的 MR(0.17% 蛋氨酸,w/w)干预可改善雄性 db/db 小鼠(2 型糖尿病模型)的外周胰岛素敏感性。值得注意的是,MR能明显改善db/db小鼠的工作记忆和长期记忆,同时还能提高PSD-95水平,减少神经炎症因子、丙二醛(MDA)和8-羟基-2'-脱氧鸟苷(8-OHdG)。我们推测这种效应可能是通过 MR 激活肝脏成纤维细胞生长因子 21(FGF21)和大脑 FGFR1/AMPK/GLUT4 信号通路来加强大脑葡萄糖代谢。为进一步阐明其机制,我们采用脑室内注射腺相关病毒的方法特异性敲除脑内的FGFR1,以验证FGFR1在MR介导的DACI中的作用。研究发现,MR 对 DACI 的积极作用被抵消,表现为认知功能下降、突触可塑性受损、神经炎症上调以及活性氧调节酶(Sod1、Sod2、Nox4)平衡。值得注意的是,FGFR1/AMPK/GLUT4 信号通路和脑葡萄糖代谢受到抑制。总之,我们的研究表明,MR 提高了外周胰岛素敏感性,通过 FGF21 激活了大脑 FGFR1/AMPK/GLUT4 信号传导,维持了大脑正常的葡萄糖代谢和氧化还原平衡,从而缓解了 DACI。这些结果为研究 MR 饮食对大脑能量代谢受损导致的认知功能障碍的影响提供了新的视角。本文章由计算机程序翻译,如有差异,请以英文原文为准。
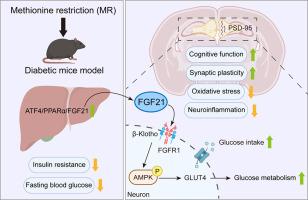
Methionine restriction alleviates diabetes-associated cognitive impairment via activation of FGF21
Glucose metabolism disturbances may result in diabetes-associated cognitive decline (DACI). Methionine restriction (MR) diet has emerged as a potential dietary strategy for managing glucose homeostasis. However, the effects and underlying mechanisms of MR on DACI have not been fully elucidated. Here, we found that a 13-week MR (0.17 % methionine, w/w) intervention starting at 8 weeks of age improved peripheral insulin sensitivity in male db/db mice, a model for type 2 diabetes. Notably, MR significantly improved working as well as long-term memory in db/db mice, accompanied by increased PSD-95 level and reduced neuroinflammatory factors, malondialdehyde (MDA), and 8-hydroxy-2′-deoxyguanosine (8-OHdG). We speculate that this effect may be mediated by MR activating hepatic fibroblast growth factor 21 (FGF21) and the brain FGFR1/AMPK/GLUT4 signaling pathway to enhance brain glucose metabolism. To further delineate the mechanism, we used intracerebroventricular injection of adeno-associated virus to specifically knock down FGFR1 in the brain to verify the role of FGFR1 in MR-mediated DACI. It was found that the positive effects of MR on DACI were offset, reflected in decreased cognitive function, impaired synaptic plasticity, upregulated neuroinflammation, and balanced enzymes regulating reactive oxygen species (Sod1, Sod2, Nox4). Of note, the FGFR1/AMPK/GLUT4 signaling pathway and brain glucose metabolism were inhibited. In summary, our study demonstrated that MR increased peripheral insulin sensitivity, activated brain FGFR1/AMPK/GLUT4 signaling through FGF21, maintained normal glucose metabolism and redox balance in the brain, and thereby alleviated DACI. These results provide new insights into the effects of MR diet on cognitive dysfunction caused by impaired brain energy metabolism.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Redox Biology
BIOCHEMISTRY & MOLECULAR BIOLOGY-
CiteScore
19.90
自引率
3.50%
发文量
318
审稿时长
25 days
期刊介绍:
Redox Biology is the official journal of the Society for Redox Biology and Medicine and the Society for Free Radical Research-Europe. It is also affiliated with the International Society for Free Radical Research (SFRRI). This journal serves as a platform for publishing pioneering research, innovative methods, and comprehensive review articles in the field of redox biology, encompassing both health and disease.
Redox Biology welcomes various forms of contributions, including research articles (short or full communications), methods, mini-reviews, and commentaries. Through its diverse range of published content, Redox Biology aims to foster advancements and insights in the understanding of redox biology and its implications.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


