拟南芥精琥珀酸裂解酶的结构揭示了丝氨酸作为催化基的作用。
IF 2.7
3区 生物学
Q3 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
精氨酸是植物体内的一种重要氨基酸,因为它不仅起着结构作用和储氮作用,还是多胺和脯氨酸等多种分子的前体。精氨酸由精琥珀酸裂解酶(ASL)生成,该酶催化精琥珀酸裂解为精氨酸和富马酸。ASL 属于富马酸裂解酶家族,尽管该家族中的许多成员都已被很好地描述,但人们对植物 ASL 却知之甚少。在这里,我们首次展示了来自模式植物拟南芥(Arabidopsis thaliana,AtASL)的 ASL 晶体结构。其中一个结构代表了 AtASL 同源四聚体的非连接形式。另一个结构是从浸泡在精琥珀酸中的晶体中获得的,它将底物或反应产物容纳在 AtASL 四聚体的四个活性位点之一。每个活性位点都位于三个相邻原体的界面上。带有配体的 AtASL 结构使我们能够详细分析酶与底物和酶与产物之间的相互作用。此外,根据我们的分析,我们描述了AtASL中对催化作用至关重要的残基。AtASL 的结构给出了 GSS 移动环从打开到关闭转变的基本原理,并指出了该环中的丝氨酸 333 对该酶的酶促作用的重要性。最后,我们通过鉴定 ASL 的特征序列图案对结构数据进行了补充。本文章由计算机程序翻译,如有差异,请以英文原文为准。
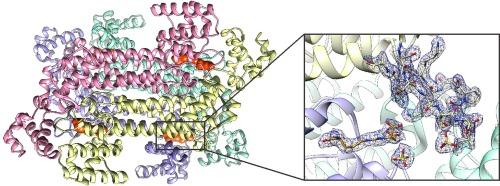
Arabidopsis thaliana argininosuccinate lyase structure uncovers the role of serine as the catalytic base
Arginine is an important amino acid in plants, as it not only plays a structural role and serves as nitrogen storage but is also a precursor for various molecules, including polyamines and proline. Arginine is produced by argininosuccinate lyase (ASL) which catalyzes the cleavage of argininosuccinate to arginine and fumarate. ASL belongs to the fumarate lyase family and while many members of this family were well-characterized, little is known about plant ASLs. Here we present the first crystal structures of ASL from the model plant, Arabidopsis thaliana (AtASL). One of the structures represents the unliganded form of the AtASL homotetramer. The other structure, obtained from a crystal soaked in argininosuccinate, accommodates the substrate or the reaction products in one of four active sites of the AtASL tetramer. Each active site is located at the interface of three neighboring protomers. The AtASL structure with ligands allowed us to analyze the enzyme-substrate and the enzyme-product interactions in detail. Furthermore, based on our analyses, we describe residues of AtASL crucial for catalysis. The structure of AtASL gives the rationale for the open-to-close transition of the GSS mobile loop and indicates the importance of serine 333 from this loop for the enzymatic action of the enzyme. Finally, we supplemented the structural data with the identification of sequence motifs characteristic for ASLs.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of structural biology
生物-生化与分子生物学
CiteScore
6.30
自引率
3.30%
发文量
88
审稿时长
65 days
期刊介绍:
Journal of Structural Biology (JSB) has an open access mirror journal, the Journal of Structural Biology: X (JSBX), sharing the same aims and scope, editorial team, submission system and rigorous peer review. Since both journals share the same editorial system, you may submit your manuscript via either journal homepage. You will be prompted during submission (and revision) to choose in which to publish your article. The editors and reviewers are not aware of the choice you made until the article has been published online. JSB and JSBX publish papers dealing with the structural analysis of living material at every level of organization by all methods that lead to an understanding of biological function in terms of molecular and supermolecular structure.
Techniques covered include:
• Light microscopy including confocal microscopy
• All types of electron microscopy
• X-ray diffraction
• Nuclear magnetic resonance
• Scanning force microscopy, scanning probe microscopy, and tunneling microscopy
• Digital image processing
• Computational insights into structure
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


