空间代谢组学发现肥厚性瘢痕相关代谢改变和潜在治疗策略:初步研究。
IF 4.5
2区 医学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
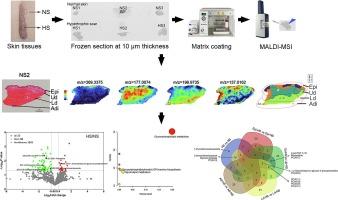
绘制增生性疤痕和周围正常皮肤组织代谢重塑的空间图,有可能加深我们对疤痕形成的理解,并有助于推进治疗干预措施。在这项研究中,我们采用基质辅助激光解吸/电离(MALDI)这种质谱成像技术,对增生性疤痕和周围正常皮肤组织切片中代谢物的层次分布进行了可视化分析。通过综合分析,在这些组织中共鉴定出 1631 种代谢物。鉴定出的前四类代谢物包括苯及其取代衍生物、杂环化合物、氨基酸及其代谢物和甘油磷脂。在肥厚性疤痕组织中,有 22 种代谢物上调,66 种代谢物下调。MetaboAnalyst 通路分析表明,甘油磷脂代谢主要与这 88 种代谢物的改变有关。随后,研究人员选择了六种代谢物,分析了它们的空间特征,并将它们分别加入原发性肥厚性瘢痕成纤维细胞的细胞培养基中。这项研究的初步结果表明,特定浓度的 1-吡咯烷甲酰胺、2-亚苄基庚醛、三油酸甘油酯、Lyso-PAF C-16 和莫索尼定能有效抑制 COL1A1、COL1A2、COL3A1 和 ACTA2 的表达。这些生物活性代谢物表现出温和无毒的特性,同时具有良好的药代动力学和药效学特性,因此很有希望用于药物开发。因此,这项研究为增生性疤痕的治疗提供了新的治疗思路。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Spatial metabolomics to discover hypertrophic scar relevant metabolic alterations and potential therapeutic strategies: A preliminary study
Spatially mapping the metabolic remodeling of hypertrophic scar and surrounding normal skin tissues has the potential to enhance our comprehension of scar formation and aid in the advancement of therapeutic interventions. In this study, we employed matrix-assisted laser desorption/ionization (MALDI), a mass spectrometry imaging technique, to visualize the hierarchical distribution of metabolites within sections of hypertrophic scar and surrounding normal skin tissues. A comprehensive analysis identified a total of 1631 metabolites in these tissues. The top four classes that were identified included benzene and substituted derivatives, heterocyclic compounds, amino acids and its metabolites, and glycerophospholipids. In hypertrophic scar tissues, 22 metabolites were upregulated and 66 metabolites were downregulated. MetaboAnalyst pathway analysis indicated that glycerophospholipid metabolism was primarily associated with these altered 88 metabolites. Subsequently, six metabolites were selected, their spatial characteristics were analyzed, and they were individually added to the cell culture medium of primary hypertrophic scar fibroblasts. The preliminary findings of this study demonstrate that specific concentrations of 1-pyrrolidinecarboxamide, 2-benzylideneheptanal, glycerol trioleate, Lyso-PAF C-16, and moxonidine effectively inhibited the expressions of COL1A1, COL1A2, COL3A1, and ACTA2. These bioactive metabolites exhibit mild and non-toxic properties, along with favorable pharmacokinetics and pharmacodynamics, making them promising candidates for drug development. Consequently, this research offers novel therapeutic insights for hypertrophic scar treatment.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Bioorganic Chemistry
生物-生化与分子生物学
CiteScore
9.70
自引率
3.90%
发文量
679
审稿时长
31 days
期刊介绍:
Bioorganic Chemistry publishes research that addresses biological questions at the molecular level, using organic chemistry and principles of physical organic chemistry. The scope of the journal covers a range of topics at the organic chemistry-biology interface, including: enzyme catalysis, biotransformation and enzyme inhibition; nucleic acids chemistry; medicinal chemistry; natural product chemistry, natural product synthesis and natural product biosynthesis; antimicrobial agents; lipid and peptide chemistry; biophysical chemistry; biological probes; bio-orthogonal chemistry and biomimetic chemistry.
For manuscripts dealing with synthetic bioactive compounds, the Journal requires that the molecular target of the compounds described must be known, and must be demonstrated experimentally in the manuscript. For studies involving natural products, if the molecular target is unknown, some data beyond simple cell-based toxicity studies to provide insight into the mechanism of action is required. Studies supported by molecular docking are welcome, but must be supported by experimental data. The Journal does not consider manuscripts that are purely theoretical or computational in nature.
The Journal publishes regular articles, short communications and reviews. Reviews are normally invited by Editors or Editorial Board members. Authors of unsolicited reviews should first contact an Editor or Editorial Board member to determine whether the proposed article is within the scope of the Journal.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


