整合乌普隆和二氧化碳穿梭策略,从醛类合成 12C- 和 13C-α 酮酸
IF 4.9
1区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
摘要
由于醛和二氧化碳这两种亲电底物之间的极性不匹配,因此很少能用二氧化碳直接羧化醛。为了应对这一挑战,我们提出了一种通过整合umpolung 和 CO2 穿梭策略从市售醛类合成 α-酮酸的连续方法。这种不含过渡金属的梭式羧化方法能将二氧化碳从三苯乙酸钾盐转移到硫代乙醛,无需处理加压二氧化碳气体或使用专门设备,同时还能提高反应的官能团耐受性。此外,使用等量或略微过量的三苯乙酸钾盐作为正式的二氧化碳供体,使其适用于对α-酮酸进行完全的 13C 标记。本文章由计算机程序翻译,如有差异,请以英文原文为准。
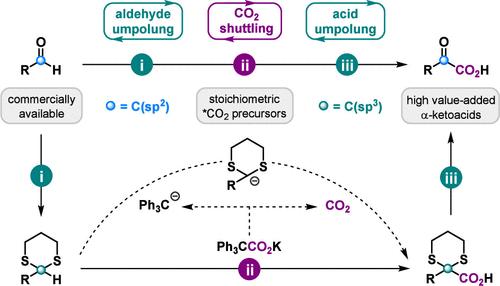
Integrating Umpolung and CO2 Shuttling Strategies for the Synthesis of 12C- and 13C-α-Ketoacids from Aldehydes
The direct carboxylation of aldehydes with CO2 is rare due to the polarity mismatch between these two electrophilic substrates. To address this challenge, we propose a sequential approach for synthesizing α-ketoacids from commercially available aldehydes by integrating umpolung and CO2 shuttling strategies. This transition metal-free shuttle carboxylation method enables the transfer of CO2 from triphenylacetic acid potassium salt to thioacetal, eliminating the need for handling pressurized CO2 gas or using specialized equipment, while also enhancing the reaction’s functional group tolerance. Furthermore, the use of stoichiometric or slightly excess amounts of triphenylacetic acid potassium salt as a formal CO2 donor makes it suitable for complete 13C labeling of α-ketoacids.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Organic Letters
化学-有机化学
CiteScore
9.30
自引率
11.50%
发文量
1607
审稿时长
1.5 months
期刊介绍:
Organic Letters invites original reports of fundamental research in all branches of the theory and practice of organic, physical organic, organometallic,medicinal, and bioorganic chemistry. Organic Letters provides rapid disclosure of the key elements of significant studies that are of interest to a large portion of the organic community. In selecting manuscripts for publication, the Editors place emphasis on the originality, quality and wide interest of the work. Authors should provide enough background information to place the new disclosure in context and to justify the rapid publication format. Back-to-back Letters will be considered. Full details should be reserved for an Article, which should appear in due course.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


