解密家族性和散发性阿尔茨海默病中淀粉样β纤维的形态差异
IF 5.3
2区 化学
Q1 CHEMISTRY, MEDICINAL
引用次数: 0
摘要
淀粉样蛋白-β(Aβ)聚集成淀粉样纤维是阿尔茨海默病(AD)的主要病理特征。Aβ 纤维可呈现多种形态,最近发现其相对数量与不同的阿兹海默症亚型(如家族性阿兹海默症和散发性阿兹海默症,分别简称为 fAD 和 sAD)有关。这两种AD亚型在发病年龄、AD相关遗传倾向和显性Aβ纤维形态上都有所不同。我们推测,这些依赖于疾病亚型的纤维形态差异可归因于纤维的内在特性和环境中的相互作用分子。通过原子离散分子动力学模拟,我们证明了与 sAD 主导的纤维形态相比,fAD 主导的形态表现出更低的纤维生长自由能垒,但稳定性也更低,从而导致与实验观察结果一致的随时间变化的种群变化。此外,我们还研究了 Bri2 BRICHOS 结构域作为可能的环境因素之一的影响。Bri2 BRICHOS 结构域与 fAD 优势纤维的结合比与 sAD 优势纤维的结合更强,这表明它能更有效地抑制 fAD 优势纤维的形成。这一结果解释了Bri2功能正常的散发性病例中sAD显性纤维形态的高发人群。另一方面,fAD 的遗传易感性可能会损害或压制 Bri2 的功能,从而导致大量与 fAD 相关的纤维形态。总之,我们的计算发现为阐明不同优势淀粉样蛋白纤维形态所带来的AD亚型提供了一个理论框架。本文章由计算机程序翻译,如有差异,请以英文原文为准。
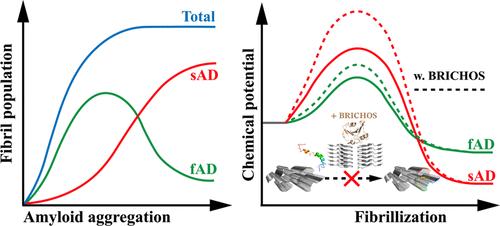
Deciphering the Morphological Difference of Amyloid-β Fibrils in Familial and Sporadic Alzheimer’s Diseases
The aggregation of amyloid-β (Aβ) into amyloid fibrils is the major pathological hallmark of Alzheimer’s disease (AD). Aβ fibrils can adopt a variety of morphologies, the relative populations of which are recently found to be associated with different AD subtypes such as familial and sporadic AD (fAD and sAD, respectively). The two AD subtypes differ in their ages of onset, AD-related genetic predispositions, and dominant Aβ fibril morphologies. We postulate that these disease subtype-dependent fibril morphology differences can be attributed to the intrinsic fibril properties and interacting molecules in the environment. Using atomistic discrete molecular dynamics simulations, we demonstrated that the fAD-dominant morphology exhibited a lower free-energy barrier for fibril growth but also a lower stability compared with the sAD-dominant fibril morphology, resulting in the time-dependent population change consistent with experimental observations. Additionally, we studied the effect of the Bri2 BRICHOS domain, an endogenous protein that has been reported to inhibit Aβ aggregation by preferential binding to fibrils, as one of the possible environmental factors. The Bri2 BRICHOS domain showed stronger binding to the fAD-dominant fibril than the sAD-dominant fibril in silico, suggesting a more effective suppression of fAD-dominant fibril formation. This result explains the high population of the sAD-dominant fibril morphology in sporadic cases with normal Bri2 functions. Genetic predisposition in fAD, on the other hand, might impair or overwhelm Bri2 functions, leading to a high population of fAD-associated fibril morphology. Together, our computational findings provide a theoretical framework for elucidating the AD subtypes entailed by distinct dominant amyloid fibril morphologies.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
9.80
自引率
10.70%
发文量
529
审稿时长
1.4 months
期刊介绍:
The Journal of Chemical Information and Modeling publishes papers reporting new methodology and/or important applications in the fields of chemical informatics and molecular modeling. Specific topics include the representation and computer-based searching of chemical databases, molecular modeling, computer-aided molecular design of new materials, catalysts, or ligands, development of new computational methods or efficient algorithms for chemical software, and biopharmaceutical chemistry including analyses of biological activity and other issues related to drug discovery.
Astute chemists, computer scientists, and information specialists look to this monthly’s insightful research studies, programming innovations, and software reviews to keep current with advances in this integral, multidisciplinary field.
As a subscriber you’ll stay abreast of database search systems, use of graph theory in chemical problems, substructure search systems, pattern recognition and clustering, analysis of chemical and physical data, molecular modeling, graphics and natural language interfaces, bibliometric and citation analysis, and synthesis design and reactions databases.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


