"极化水分子之刃 "是水解酶催化调节的关键
IF 5.6
2区 化学
Q1 CHEMISTRY, MEDICINAL
引用次数: 0
摘要
天冬氨酸蛋白酶催化的水解是许多生物过程中的关键反应。然而,锚定水分子和统一多种催化途径仍然是重大挑战。因此,分子设计通常会在提高底物特异性方面做出妥协。利用我们自主开发的可极化点电荷(PPC)力场,我们首次确定了极化在胃蛋白酶水解酶中的重要作用。为了稳定地锚定在活性位点上,水应该被强烈极化,电荷高于-0.94e。在这种极化作用的诱导下,胃蛋白酶被证明支持三种一般碱/一般酸途径,并偏向于以吉二醇-中间体为基础的途径。因此,我们提出了用于合理酶设计的 "极化水分子刀片 "模型,强调了攻击水和攻击羰基的极化对于增强水解作用至关重要。突变体 D290Q 和 S172P 的活性分别提高了 191.23% 和 324.70%。突变体中水、羰基和相关亲核攻击距离的极化得到了改善,这再次证实了极化在改善水解过程中的关键作用。这项研究为水解酶的分析和改造提供了一个新的视角。本文章由计算机程序翻译,如有差异,请以英文原文为准。
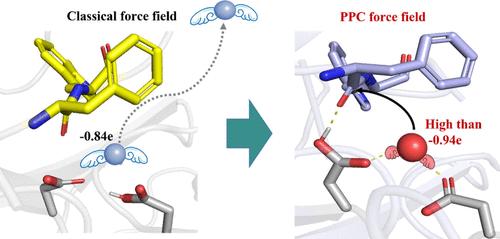
“Blade of Polarized Water Molecule” Is the Key to Hydrolase Catalysis Regulation
Hydrolysis catalyzed by aspartic proteases is a crucial reaction in many biological processes. However, anchoring water molecules and unifying multiple catalytic pathways remain significant challenges. Consequently, molecular design often compromises by focusing on enhancing substrate specificity. Using our self-developed polarizable point charge (PPC) force field, we determined the significant role of polarization in the hydrolase of pepsin for the first time. To be stably anchored in the active site, the water should be intensely polarized with a charge higher than −0.94e. Induced by this polarization, the pepsin was shown to support three general base/general acid pathways, with a preference for the gemdiol-intermediate-based pathway. Consequently, we proposed the “Blade of Polarized Water Molecule” model for rational enzyme design, highlighting that the polarization of both the attacking water and the attacked carbonyl is crucial for enhancing hydrolysis. Mutants D290Q and S172P showed activity enhancements of 191.23% and 324.70%, respectively. The improved polarization of water, carbonyl, and relevant nucleophilic attack distances in the mutants reaffirmed the crucial role of polarization in improving hydrolysis. This study provides a new perspective on hydrolase analysis and modification.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
9.80
自引率
10.70%
发文量
529
审稿时长
1.4 months
期刊介绍:
The Journal of Chemical Information and Modeling publishes papers reporting new methodology and/or important applications in the fields of chemical informatics and molecular modeling. Specific topics include the representation and computer-based searching of chemical databases, molecular modeling, computer-aided molecular design of new materials, catalysts, or ligands, development of new computational methods or efficient algorithms for chemical software, and biopharmaceutical chemistry including analyses of biological activity and other issues related to drug discovery.
Astute chemists, computer scientists, and information specialists look to this monthly’s insightful research studies, programming innovations, and software reviews to keep current with advances in this integral, multidisciplinary field.
As a subscriber you’ll stay abreast of database search systems, use of graph theory in chemical problems, substructure search systems, pattern recognition and clustering, analysis of chemical and physical data, molecular modeling, graphics and natural language interfaces, bibliometric and citation analysis, and synthesis design and reactions databases.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


