xCT降解引发的脂质过氧化是慢性阻塞性肺病中气敏剂D介导的脓毒症的诱因。
IF 10.7
1区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
背景脓毒血症是一种调节性坏死的炎症形式,与慢性阻塞性肺病(COPD)的发病机制有关。方法在体外,人支气管上皮细胞(Beas-2b 细胞)暴露于香烟烟雾提取物(CSE)24 小时。在体内,小鼠暴露于香烟烟雾(CS)4 周。结果给予铁前列素-1(Fer-1)(一种抑制脂质过氧化的铁氧化抑制剂)可显著降低 CSE 对 Beas-2b 细胞的细胞毒性,并减轻 CS 诱导的慢性阻塞性肺病小鼠的炎症渗出、肺损伤和粘液分泌过多。Fer-1 可抑制 CS 在体外和体内介导的 gasdermin D(GSDMD)热昏迷。然而,在 Beas-2b 细胞和小鼠肺上皮细胞中,条件性敲除 xCT(脂质过氧化负调控因子)抑制了 xCT/GPx4 轴,导致香烟烟雾暴露时脂质过氧化和 GSDMD 介导的热猝死更为严重。此外,我们还发现 CS 通过泛素蛋白酶体系统(UPS)促进了 xCT 的降解,而 MG132 能显著抑制 xCT 的降解并下调热蛋白沉积相关蛋白的表达。本文章由计算机程序翻译,如有差异,请以英文原文为准。
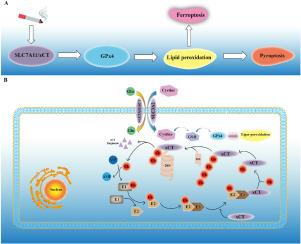
Lipid peroxidation triggered by the degradation of xCT contributes to gasdermin D-mediated pyroptosis in COPD
Background
Pyroptosis is an inflammatory form of regulated necrosis that has been implicated in the pathogenesis of chronic obstructive pulmonary disease (COPD). However, the role of lipid peroxidation in pyroptosis and its underlying mechanisms in COPD remain unclear.
Methods
In vitro, human bronchial epithelial cells (Beas-2b cells) were exposed to cigarette smoke extract (CSE) for 24 h. In vivo, mice were exposed to cigarette smoke (CS) for 4 weeks. To investigate the role of xCT, we used siRNA and AAV6 to conditionally knock down xCT in vitro and in vivo, respectively.
Results
The administration of ferrostatin-1 (Fer-1), a ferroptosis inhibitor that inhibits lipid peroxidation, significantly reduced the cytotoxicity of CSE to Beas-2b cells and mitigated inflammatory exudation, lung injury and mucus hypersecretion in mice with CS-induced COPD. Fer-1 suppressed gasdermin D (GSDMD)-mediated pyroptosis caused by CS in vitro and in vivo. However, in Beas-2b cells and the lung epithelial cells of mice, conditional knockdown of xCT (a negative regulatory factor of lipid peroxidation) inhibited the xCT/GPx4 axis, leading to more severe lipid peroxidation and GSDMD-mediated pyroptosis during cigarette smoke exposure. Moreover, we found that CS promoted the degradation of xCT through the ubiquitin proteasome system (UPS) and that treatment with MG132 significantly inhibited the degradation of xCT and downregulated the expression of pyroptosis-related proteins.
Conclusion
The results of this study suggested that the ubiquitination-mediated degradation of xCT drives GSDMD-mediated pyroptosis in COPD and is a potential therapeutic target for COPD.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Redox Biology
BIOCHEMISTRY & MOLECULAR BIOLOGY-
CiteScore
19.90
自引率
3.50%
发文量
318
审稿时长
25 days
期刊介绍:
Redox Biology is the official journal of the Society for Redox Biology and Medicine and the Society for Free Radical Research-Europe. It is also affiliated with the International Society for Free Radical Research (SFRRI). This journal serves as a platform for publishing pioneering research, innovative methods, and comprehensive review articles in the field of redox biology, encompassing both health and disease.
Redox Biology welcomes various forms of contributions, including research articles (short or full communications), methods, mini-reviews, and commentaries. Through its diverse range of published content, Redox Biology aims to foster advancements and insights in the understanding of redox biology and its implications.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


