生物界面上 α-突触核蛋白四聚体的构象选择
IF 5.3
2区 化学
Q1 CHEMISTRY, MEDICINAL
引用次数: 0
摘要
控制α-突触核蛋白(αS)寡聚体的多态组装对于改变帕金森病(PD)中涉及的毒性蛋白质聚集至关重要。αS四聚体与细胞膜的相互作用是一个潜在的中介,它可能调节易聚集的无序单体与抗聚集的螺旋四聚体之间的动态平衡。在这里,我们建立了四聚体-细胞相互作用的各种模型,并将超分子-生物界面的结构-功能关系与现有的实验数据进行了比较。模型预测紧凑型 αS 四聚体会优先与高电荷膜表面相互作用,这可能会进一步稳定这种抗聚集构象。在电荷适中的膜上,扩展结构更受青睐。除表面电荷外,曲率也会影响四聚体的热力学稳定性和聚集,通过与强负电荷胶束的区域特异性相互作用,有可能选择性地分离四聚体,从而阻止进一步聚集。我们的建模数据集凸显了多种有益的纳米生物相互作用,可重新定向生物分子的组装,支持基于脂质介导的构象选择和抑制作用的新治疗方法。本文章由计算机程序翻译,如有差异,请以英文原文为准。
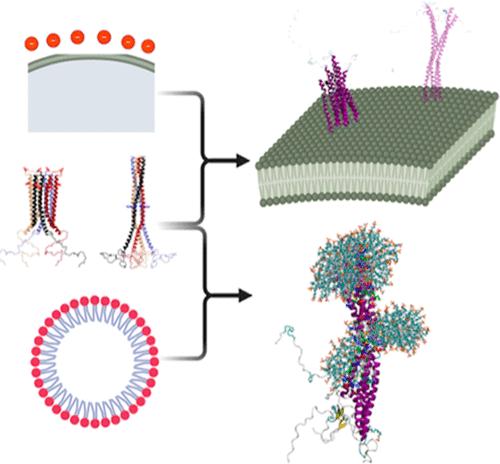
Conformational Selection of α-Synuclein Tetramers at Biological Interfaces
Controlling the polymorphic assemblies of α-synuclein (αS) oligomers is crucial to reroute toxic protein aggregation implicated in Parkinson’s disease (PD). One potential mediator is the interaction of αS tetramers with cell membranes, which may regulate the dynamic balance between aggregation-prone disordered monomers and aggregation-resistant helical tetramers. Here, we model diverse tetramer–cell interactions and compare the structure–function relations at the supramolecular–biological interface with available experimental data. The models predict preferential interaction of compact αS tetramers with highly charged membrane surfaces, which may further stabilize this aggregation-resistant conformer. On moderately charged membranes, extended structures are preferred. In addition to surface charge, curvature influences tetramer thermodynamic stability and aggregation, with potential for selective isolation of tetramers via regio-specific interactions with strongly negatively charged micelles that screen further aggregation. Our modeling data set highlights diverse beneficial nano–bio interactions to redirect biomolecule assembly, supporting new therapeutic approaches for PD based on lipid-mediated conformational selection and inhibition.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
9.80
自引率
10.70%
发文量
529
审稿时长
1.4 months
期刊介绍:
The Journal of Chemical Information and Modeling publishes papers reporting new methodology and/or important applications in the fields of chemical informatics and molecular modeling. Specific topics include the representation and computer-based searching of chemical databases, molecular modeling, computer-aided molecular design of new materials, catalysts, or ligands, development of new computational methods or efficient algorithms for chemical software, and biopharmaceutical chemistry including analyses of biological activity and other issues related to drug discovery.
Astute chemists, computer scientists, and information specialists look to this monthly’s insightful research studies, programming innovations, and software reviews to keep current with advances in this integral, multidisciplinary field.
As a subscriber you’ll stay abreast of database search systems, use of graph theory in chemical problems, substructure search systems, pattern recognition and clustering, analysis of chemical and physical data, molecular modeling, graphics and natural language interfaces, bibliometric and citation analysis, and synthesis design and reactions databases.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


