锰催化环氧化物与醇的开环和烷基化反应,通过借氢获得β-烷基仲醇
IF 4.7
1区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
摘要
本文报告的是一种定义明确的双齿几何约束吡啶腙锰配合物,用于高效和高选择性开环&;环氧化物与醇的烷基化反应,生成 烷基化仲醇。该方法的底物范围很广,包括芳香族和脂肪族环氧化物与(杂)芳香族和脂肪族一级/二级醇。控制和标记实验表明,该反应是通过环氧化物与醇的还原性开环反应和 借氢途径使醇烷基化进行的。本文章由计算机程序翻译,如有差异,请以英文原文为准。
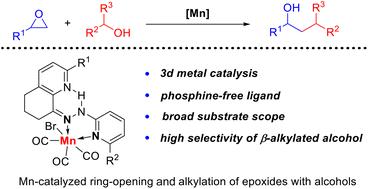
Mn-catalyzed ring-opening and alkylation of epoxides with alcohols for accessing β-alkylated secondary alcohols via borrowing hydrogen†
Reported herein is a well-defined bidentate geometry-constrained pyridyl hydrazone manganese complex for an efficient and highly selective ring-opening and alkylation of epoxides with alcohols to deliver β-alkylated secondary alcohols. A broad substrate scope was shown, involving aromatic and aliphatic epoxides with both (hetero)aromatic and aliphatic primary/secondary alcohols. Control and labelling experiments showed that the reaction underwent a reductive ring-opening of epoxide with alcohol and β-alkylation of alcohol via a borrowing hydrogen pathway.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Organic Chemistry Frontiers
CHEMISTRY, ORGANIC-
CiteScore
7.90
自引率
11.10%
发文量
686
审稿时长
1 months
期刊介绍:
Organic Chemistry Frontiers is an esteemed journal that publishes high-quality research across the field of organic chemistry. It places a significant emphasis on studies that contribute substantially to the field by introducing new or significantly improved protocols and methodologies. The journal covers a wide array of topics which include, but are not limited to, organic synthesis, the development of synthetic methodologies, catalysis, natural products, functional organic materials, supramolecular and macromolecular chemistry, as well as physical and computational organic chemistry.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


