非活性 Parp2 通过阻断红细胞中与复制相关的缺口连接,导致 Tp53 依赖性致死性贫血
IF 14.5
1区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
聚(ADP-核糖)聚合酶(PARP)1 和 2 酶抑制剂(PARPi)是一种很有前景的癌症治疗方法。但最近,这些药物的使用受到了不明原因的严重贫血和治疗相关性白血病的阻碍。除了酶抑制作用外,PARPi 还能将 PARP1 和 2 困在 DNA 病变处。我们在此报告,与正常发育的 Parp2-/-小鼠不同,表达无催化活性的 Parp2(E534A 和 Parp2EA/EA)的小鼠在子宫内会出现 Tp53 和 Chk2 依赖性红细胞生成障碍,这与 Lig1-/- 小鼠的情况如出一辙。虽然 DNA 损伤主要激活 PARP1,但我们证明 DNA 复制能强有力地激活 PARP2。PARP2会被5′-磷酸化缺口(5′p-缺口)选择性地招募和激活,包括被连接酶1(Lig1)和Lig3分解的冈崎片段之间的缺口。非活性 PARP2(而非其活性形式或缺失)会阻碍 Lig1 和 Lig3 介导的连接,导致剂量依赖性复制叉崩溃,这对具有超快复制叉的红细胞是有害的。PARP2 在 5′p 缺口处的这种 PARylation 依赖性结构功能解释了 PARP2 失活对红细胞生成的不利影响,揭示了 PARPi 诱导的贫血和 TP53/CHK2 缺失的选择。本文章由计算机程序翻译,如有差异,请以英文原文为准。
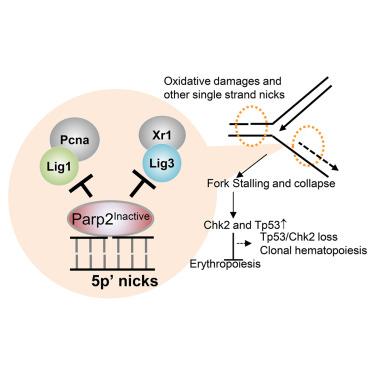
Inactive Parp2 causes Tp53-dependent lethal anemia by blocking replication-associated nick ligation in erythroblasts
Poly (ADP-ribose) polymerase (PARP) 1 and 2 enzymatic inhibitors (PARPi) are promising cancer treatments. But recently, their use has been hindered by unexplained severe anemia and treatment-related leukemia. In addition to enzymatic inhibition, PARPi also trap PARP1 and 2 at DNA lesions. Here we report that, unlike Parp2−/− mice, which develop normally, mice expressing catalytically inactive Parp2 (E534A and Parp2EA/EA) succumb to Tp53- and Chk2-dependent erythropoietic failure in utero, mirroring Lig1−/− mice. While DNA damage mainly activates PARP1, we demonstrate that DNA replication activates PARP2 robustly. PARP2 is selectively recruited and activated by 5′-phosphorylated nicks (5′p-nicks), including those between Okazaki fragments, resolved by ligase 1 (Lig1) and Lig3. Inactive PARP2, but not its active form or absence, impedes Lig1- and Lig3-mediated ligation, causing dose-dependent replication fork collapse, which is detrimental to erythroblasts with ultra-fast forks. This PARylation-dependent structural function of PARP2 at 5′p-nicks explains the detrimental effects of PARP2 inactivation on erythropoiesis, shedding light on PARPi-induced anemia and the selection for TP53/CHK2 loss.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Molecular Cell
生物-生化与分子生物学
CiteScore
26.00
自引率
3.80%
发文量
389
审稿时长
1 months
期刊介绍:
Molecular Cell is a companion to Cell, the leading journal of biology and the highest-impact journal in the world. Launched in December 1997 and published monthly. Molecular Cell is dedicated to publishing cutting-edge research in molecular biology, focusing on fundamental cellular processes. The journal encompasses a wide range of topics, including DNA replication, recombination, and repair; Chromatin biology and genome organization; Transcription; RNA processing and decay; Non-coding RNA function; Translation; Protein folding, modification, and quality control; Signal transduction pathways; Cell cycle and checkpoints; Cell death; Autophagy; Metabolism.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


