受亲核性标度启发的 TCFH-NMI 酮合成技术
IF 5
1区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
摘要
N,N,N′,N′-四甲基氯甲胺鎓六氟磷酸盐(TCFH)和 N-甲基咪唑(NMI)能使羧酸与胺、醇和硫醇发生简便实用的反应,生成酰胺、酯和硫代酯。为了开发一种在室温下直接用 TCFH-NMI 从羧酸合成酮的温和方法,我们使用 Mayr 亲核性标度来比较合格亲核物与潜在碳中心亲核物的 N 值,当 N ≥ 10 时,确定吡咯和吲哚为成功的底物。本文章由计算机程序翻译,如有差异,请以英文原文为准。
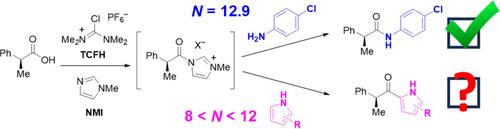
TCFH–NMI Ketone Synthesis Inspired by Nucleophilicity Scales
N,N,N′,N′-Tetramethylchloroformamidinium hexafluorophosphate (TCFH) and N-methylimidazole (NMI) enable the facile and practical reaction of carboxylic acids with amines, alcohols, and thiols to form amides, esters, and thioesters. To develop a mild synthesis of ketones with TCFH–NMI directly from carboxylic acids at room temperature, the Mayr nucleophilicity scale was used to compare the N values of competent nucleophiles to potential carbon-centered nucleophiles, identifying pyrroles and indoles as successful substrates when N ≥ 10.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Organic Letters
化学-有机化学
CiteScore
9.30
自引率
11.50%
发文量
1607
审稿时长
1.5 months
期刊介绍:
Organic Letters invites original reports of fundamental research in all branches of the theory and practice of organic, physical organic, organometallic,medicinal, and bioorganic chemistry. Organic Letters provides rapid disclosure of the key elements of significant studies that are of interest to a large portion of the organic community. In selecting manuscripts for publication, the Editors place emphasis on the originality, quality and wide interest of the work. Authors should provide enough background information to place the new disclosure in context and to justify the rapid publication format. Back-to-back Letters will be considered. Full details should be reserved for an Article, which should appear in due course.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


