NHC 催化的 Umpolung 驱动分子内环化作用生成 3,4-环庚酮嵌合四环吲哚衍生物
IF 5
1区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
摘要
在这封信中,我们揭示了在生成四环吲哚衍生物时,N-杂环碳烯(NHC)有机催化剂在引入环庚烷成环过程中的umpolung反应活性的开发和利用。通过 NHC 催化的分子内乙烯基斯泰特、斯泰特、安息香和正式交叉脱氢偶联转化,以良好到极佳的收率构建了 3,4-环庚烷化吲哚衍生物。所开发的方案利用廉价催化剂,具有操作简单、原子经济、克级合成和合成后可用性等特点。本文章由计算机程序翻译,如有差异,请以英文原文为准。
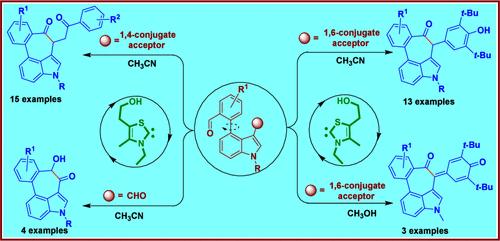
NHC-Catalyzed Umpolung Driven Intramolecular Cyclizations for the Generation of 3,4-Cycloheptanone Annulated Tetracyclic Indole Derivatives
In this Letter, we disclose the development and exploitation of umpolung reactivity of N-heterocyclic carbene (NHC) organocatalysts in generating tetracyclic indole derivatives with the introduction of a cycloheptane ring forming event. The NHC-catalyzed intramolecular vinylogous Stetter, Stetter, benzoin, and formal cross-dehydrogenative coupling transformations have been executed, enabling the construction of 3,4-cycloheptannulated indole derivatives in good to excellent yields. The developed protocols utilize an inexpensive catalyst and feature operational simplicity, atom economy, gram-scale syntheses, and postsynthetic availability.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Organic Letters
化学-有机化学
CiteScore
9.30
自引率
11.50%
发文量
1607
审稿时长
1.5 months
期刊介绍:
Organic Letters invites original reports of fundamental research in all branches of the theory and practice of organic, physical organic, organometallic,medicinal, and bioorganic chemistry. Organic Letters provides rapid disclosure of the key elements of significant studies that are of interest to a large portion of the organic community. In selecting manuscripts for publication, the Editors place emphasis on the originality, quality and wide interest of the work. Authors should provide enough background information to place the new disclosure in context and to justify the rapid publication format. Back-to-back Letters will be considered. Full details should be reserved for an Article, which should appear in due course.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


