基质雄激素信号调节支持前列腺发育和肿瘤发生的重要龛位。
IF 6.9
1区 医学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
雄激素和雄激素受体(AR)介导的信号通路对前列腺的发育、形态发生、生长和再生至关重要。早期的组织重组实验表明,AR缺陷的尿窦间充质与完整的尿窦上皮结合后不能发育成前列腺,这证明了间充质AR在前列腺发育中的干细胞位。雄激素信号对前列腺在出生后阶段的成熟和生长仍然至关重要。重要的是,大多数原发性前列腺癌(PCa)细胞表达AR,AR的异常激活直接促进了PCa的发育、生长和恶化。因此,针对 PCa 细胞中 AR 的雄激素剥夺疗法(ADT)是治疗晚期 PCa 的主要方法。然而,这种疗法最终还是失败了,导致了阉割耐药 PCa 的发生,这是一种无法治愈的疾病。鉴于这些临床挑战,需要重新评估致癌 AR 的作用,以开发新的有效疗法。最近,研究发现基质 AR 在调控前列腺发育和肿瘤发生中发挥着重要的生态位作用。在此,我们总结了有关基质 AR 龛及其与前列腺上皮相互作用的最新发现。结合新出现的临床和实验证据,我们特别讨论了有关基质AR在肿瘤龛中作用的几个重要且长期未解的问题,并强调了通过联合靶向上皮和基质AR治疗晚期PCa的未来治疗策略。本文章由计算机程序翻译,如有差异,请以英文原文为准。
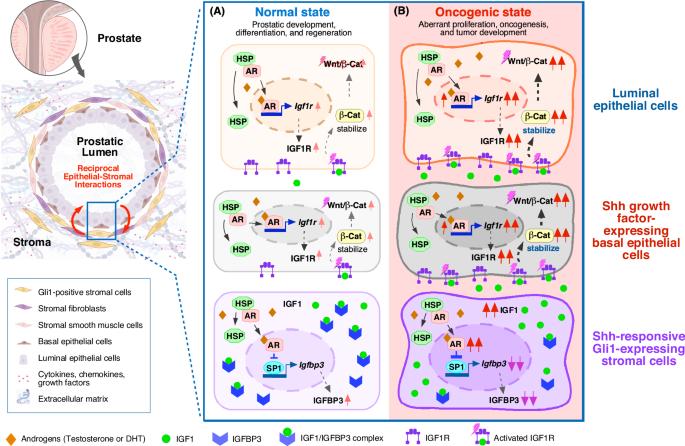
Stromal androgen signaling governs essential niches in supporting prostate development and tumorigenesis
Androgens and androgen receptor (AR) mediated signaling pathways are essential for prostate development, morphogenesis, growth, and regeneration. Early tissue recombination experiments showed that AR-deficient urogenital sinus mesenchyme combined with intact urogenital sinus epithelium failed to develop into a prostate, demonstrating a stem cell niche for mesenchymal AR in prostatic development. Androgen signaling remains critical for prostate maturation and growth during postnatal stages. Importantly, most primary prostate cancer (PCa) cells express the AR, and aberrant activation of AR directly promotes PCa development, growth, and progression. Therefore, androgen deprivation therapy (ADT) targeting the AR in PCa cells is the main treatment for advanced PCa. However, it eventually fails, leading to the development of castration-resistant PCa, an incurable disease. Given these clinical challenges, the oncogenic AR action needs to be reevaluated for developing new and effective therapies. Recently, an essential niche role of stromal AR was identified in regulating prostate development and tumorigenesis. Here, we summarize the latest discoveries of stromal AR niches and their interactions with prostatic epithelia. In combination with emerging clinical and experimental evidence, we specifically discuss several important and long-term unanswered questions regarding tumor niche roles of stromal AR and highlight future therapeutic strategies by co-targeting epithelial and stromal AR for treating advanced PCa.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Oncogene
医学-生化与分子生物学
CiteScore
15.30
自引率
1.20%
发文量
404
审稿时长
1 months
期刊介绍:
Oncogene is dedicated to advancing our understanding of cancer processes through the publication of exceptional research. The journal seeks to disseminate work that challenges conventional theories and contributes to establishing new paradigms in the etio-pathogenesis, diagnosis, treatment, or prevention of cancers. Emphasis is placed on research shedding light on processes driving metastatic spread and providing crucial insights into cancer biology beyond existing knowledge.
Areas covered include the cellular and molecular biology of cancer, resistance to cancer therapies, and the development of improved approaches to enhance survival. Oncogene spans the spectrum of cancer biology, from fundamental and theoretical work to translational, applied, and clinical research, including early and late Phase clinical trials, particularly those with biologic and translational endpoints.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


