雄激素消退疗法诱导 CXCL8 调节 mTORC1/SREBP2 依赖性胆固醇生物合成,支持雄激素受体阴性前列腺癌细胞的进展。
IF 7.3
1区 医学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
雄激素消退疗法能有效抑制雄激素受体(AR)阳性(AR+)前列腺癌(PCa)细胞亚型,但却导致AR阴性(AR-)PCa细胞亚型的增加。本研究旨在探讨导致细胞类型比例变化的机制,并确定 CXCL8 是 AR- PCa 细胞的合成基本效应因子。研究发现,AR- PCa 细胞易受 CXCL8 的消耗或抑制,从而影响其存活。从机理上讲,雄激素凋亡疗法抑制了AR信号传导,导致CXCL8基因转录上调。CXCL8 反过来又激活了 mTORC1 通路,后者通过激活固醇调节元件结合蛋白-2(SREBP2)增加了新胆固醇的合成。这些结果表明,在雄激素消减疗法下,CXCL8-mTORC1-SREBP2 轴促使 AR- PCa 细胞的致瘤性增强。本文章由计算机程序翻译,如有差异,请以英文原文为准。
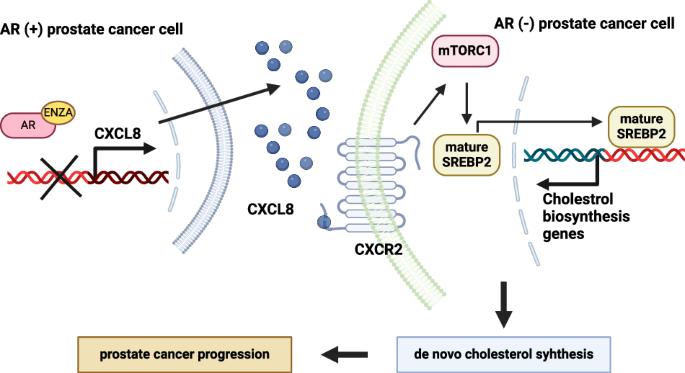
Androgen-ablative therapies inducing CXCL8 regulates mTORC1/SREBP2-dependent cholesterol biosynthesis to support progression of androgen receptor negative prostate cancer cells
Treatment with androgen-ablative therapies effectively inhibited androgen receptor (AR)-positive (AR+) prostate cancer (PCa) cell subtypes, but it resulted in an increase in AR-negative (AR−) PCa cell subtypes. The present study aimed to investigate the debated mechanisms responsible for the changing proportion of cell types, identifying CXCL8 as a synthetic essential effector of AR− PCa cells. AR− PCa cells were found to be susceptible to CXCL8 depletion or inhibition, which impaired their survival. Mechanistically, androgen-ablative therapies resulted in the suppression of AR signaling, leading to the upregulation of CXCL8 gene transcription. CXCL8, in turn, activated the mTORC1 pathway, which increased de novo cholesterol synthesis by activating sterol regulatory element-binding protein-2 (SREBP2). Together, these results suggested that the CXCL8-mTORC1-SREBP2 axis contributed to the exacerbation of tumorigenicity in AR− PCa cells under androgen-ablative therapies.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Oncogene
医学-生化与分子生物学
CiteScore
15.30
自引率
1.20%
发文量
404
审稿时长
1 months
期刊介绍:
Oncogene is dedicated to advancing our understanding of cancer processes through the publication of exceptional research. The journal seeks to disseminate work that challenges conventional theories and contributes to establishing new paradigms in the etio-pathogenesis, diagnosis, treatment, or prevention of cancers. Emphasis is placed on research shedding light on processes driving metastatic spread and providing crucial insights into cancer biology beyond existing knowledge.
Areas covered include the cellular and molecular biology of cancer, resistance to cancer therapies, and the development of improved approaches to enhance survival. Oncogene spans the spectrum of cancer biology, from fundamental and theoretical work to translational, applied, and clinical research, including early and late Phase clinical trials, particularly those with biologic and translational endpoints.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


