通过靶向 Lamp2a 激活伴侣介导的自噬,对脑出血诱导的神经元损伤发挥神经保护作用
IF 4.6
2区 医学
Q1 NEUROSCIENCES
引用次数: 0
摘要
脑内出血(ICH)是一种常见的破坏性中风,发病率高,预后严重。星形胶质细胞引发的炎症级联反应在 ICH 后的继发性脑损伤(SBI)中起着关键作用,导致细胞死亡等有害影响。然而,目前尚缺乏有效的干预策略。本研究旨在探讨 ICH 后星形胶质细胞级联反应的作用,并确定潜在的干预目标。我们利用 GSE216607 和 GSE206971 数据库进行分析,建立了小鼠自体血模型。首先,我们的研究揭示了脑内出血(ICH)后自噬通路的显著激活,主要定位于星形胶质细胞的伴侣介导的自噬(CMA)关键因子 Lamp2a 明显上调。此外,Lamp2a 的下调导致 A1 反应性星形胶质细胞显著增加,同时导致小鼠 ICH 后髓鞘覆盖面积减少、神经元损伤加重、运动和感觉障碍加剧以及神经系统评分降低。相反,CMA 的激活剂 CA77.1 可以逆转 ICH 引起的 A1 反应性星形胶质细胞增加、髓鞘损伤、神经元死亡和神经行为障碍。总之,激活 ICH 后的星形胶质细胞 CMA 可发挥神经保护作用。Lamp2a 是治疗 ICH 后的一个很有前景的治疗靶点。本文章由计算机程序翻译,如有差异,请以英文原文为准。
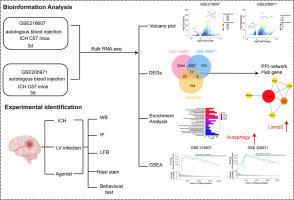
Activation of chaperone-mediated autophagy exerting neuroprotection effect on intracerebral hemorrhage-induced neuronal injury by targeting Lamp2a
Intracerebral hemorrhage (ICH) is a common and devastating type of stroke, marked by significant morbidity and a grim prognosis. The inflammation cascade triggered by astrocytes plays a critical role in secondary brain injury (SBI) following ICH, leading to detrimental effects such as cell death. However, effective intervention strategies are currently lacking. This study aims to investigate the role of the astrocyte cascade reaction following ICH and identify potential intervention targets. Utilizing the GSE216607 and GSE206971 databases for analysis, we established a mouse autologous blood model. Firstly, our research revealed a significant activation of the autophagy pathway following intracerebral hemorrhage (ICH), with a notable upregulation of Lamp2a, a key factor in chaperone-mediated autophagy (CMA), primarily localized in astrocytes. Additionally, the downregulation of Lamp2a resulted in a significant augmentation of A1 reactive astrocytes, concomitant with a reduction in myelin coverage area, heightened neuronal injury, exacerbated motor and sensory deficits, and diminished neurological scores after ICH in mice. Conversely, CA77.1, an activator of CMA, could reverse ICH-induced augmentation of A1 reactive astrocytes, myelin damage, neuronal death, and neurobehavioral disorders. In conclusion, the activation of astrocyte CMA following ICH can exert neuroprotective effects. Lamp2a represents a promising therapeutic target for post-ICH treatment.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Experimental Neurology
医学-神经科学
CiteScore
10.10
自引率
3.80%
发文量
258
审稿时长
42 days
期刊介绍:
Experimental Neurology, a Journal of Neuroscience Research, publishes original research in neuroscience with a particular emphasis on novel findings in neural development, regeneration, plasticity and transplantation. The journal has focused on research concerning basic mechanisms underlying neurological disorders.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


