经典同型胱氨酸尿症酶替代疗法的作用机制和硫醇稳态对疗效的影响。
IF 10.7
1区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
胱硫醚-β-合成酶(CBS)缺乏症导致的同型半胱氨酸尿症(HCU)以血浆和组织中同型半胱氨酸水平升高为特征。目前尚无根治方法,但 HCU 通常可通过限制蛋氨酸/蛋白质摄入和补充维生素 B6 来控制。基于人类 CBS 的酶替代疗法(ERT)已经开发出来,并在多个小鼠模型中显示出显著疗效,通过将血浆总同型半胱氨酸降至临床相关的 100 μM 临界值以下,纠正了 HCU 表型。由于同型半胱氨酸的反应性会促进二硫化物的形成和蛋白质的结合,而 ERT 无法使血浆中的同型半胱氨酸总含量恢复正常,因此 ERT 在 HCU 中的作用机制仍有待进一步研究。在这里,我们发现只有还原型同型半胱氨酸才能作为 CBS 的底物,而它的可用性限制了 CBS 降解同型半胱氨酸的能力。我们还证明,细胞输出的同型半胱氨酸是还原型的,在培养基中被 CBS 有效代谢。在我们的细胞模型中,丝氨酸(CBS 的辅助底物)的可用性并不是限制因素。生物还原剂(如 N-乙酰半胱氨酸、MESNA 或半胱胺)增加了还原型同型半胱氨酸的可用性,从而促进了随后基于 CBS 的消除。在转基因 I278T HCU 小鼠模型中,服用生物还原剂可显著增加血浆中蛋白未结合的同型半胱氨酸的比例,从而提高联合服用基于 CBS 的 ERT 的疗效,血浆总同型半胱氨酸水平的显著降低就证明了这一点。这些结果阐明了基于 CBS 的 ERT 的作用机制,并揭示了进一步提高其疗效的新型药理学方法。本文章由计算机程序翻译,如有差异,请以英文原文为准。
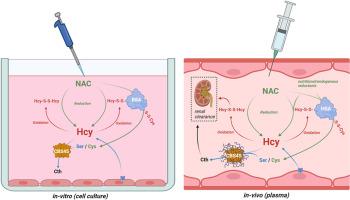
Mechanism of action and impact of thiol homeostasis on efficacy of an enzyme replacement therapy for classical homocystinuria
Homocystinuria (HCU) due to cystathionine beta-synthase (CBS) deficiency is characterized by elevated plasma and tissue homocysteine levels. There is no cure, but HCU is typically managed by methionine/protein restriction and vitamin B6 supplementation. Enzyme replacement therapy (ERT) based on human CBS has been developed and has shown significant efficacy correcting HCU phenotype in several mouse models by bringing plasma total homocysteine below the clinically relevant 100 μM threshold. As the reactive nature of homocysteine promotes disulfide formation and protein binding, and ERT is unable to normalize plasma total homocysteine levels, the mechanism of action of ERT in HCU remains to be further characterized. Here we showed that only a reduced homocysteine serves as a substrate for CBS and its availability restricts the homocysteine-degrading capacity of CBS. We also demonstrated that cells export homocysteine in its reduced form, which is efficiently metabolized by CBS in the culture medium. Availability of serine, a CBS co-substrate, was not a limiting factor in our cell-based model. Biological reductants, such as N-acetylcysteine, MESNA or cysteamine, increased the availability of the reduced homocysteine and thus promoted its subsequent CBS-based elimination. In a transgenic I278T mouse model of HCU, administration of biological reductants significantly increased the proportion of protein-unbound homocysteine in plasma, which improved the efficacy of the co-administered CBS-based ERT, as evidenced by significantly lower plasma total homocysteine levels. These results clarify the mechanism of action of CBS-based ERT and unveil novel pharmacological approaches to further increase its efficacy.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Redox Biology
BIOCHEMISTRY & MOLECULAR BIOLOGY-
CiteScore
19.90
自引率
3.50%
发文量
318
审稿时长
25 days
期刊介绍:
Redox Biology is the official journal of the Society for Redox Biology and Medicine and the Society for Free Radical Research-Europe. It is also affiliated with the International Society for Free Radical Research (SFRRI). This journal serves as a platform for publishing pioneering research, innovative methods, and comprehensive review articles in the field of redox biology, encompassing both health and disease.
Redox Biology welcomes various forms of contributions, including research articles (short or full communications), methods, mini-reviews, and commentaries. Through its diverse range of published content, Redox Biology aims to foster advancements and insights in the understanding of redox biology and its implications.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


