激酶 CK2 激活段的错义突变会导致 Okur-Chung 神经发育障碍,并改变海马谷氨酸能突触。
IF 9.6
1区 医学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
通过外显子组测序,人们发现了自闭症单基因型的致病基因,其中包括2016年发现的编码激酶CK2催化亚基的基因CSNK2A1,该基因将激酶与奥库尔-郑神经发育综合征(OCNDS)联系起来,后者是一种新描述的神经发育疾病,许多症状与自闭症谱系障碍相似。迄今为止,还没有这种疾病的临床前模型。在这里,我们描述了一种在 CK2 α 亚基激活区段携带 K198R 突变的基因敲入小鼠模型。该区域是突变热点,占患者总数的三分之一。这些小鼠表现出的行为表型与患者的症状如出一辙。同型基因敲入小鼠在妊娠中期死亡,而杂合基因敲入小鼠出生时的泯灭率仅为预期的一半,体重和体型均小于野生型同窝小鼠。杂合子基因敲入小鼠在认知和记忆评估范式中表现出改变、刻板印象增强、昼夜节律活动模式改变以及筑巢行为。脑组织磷酸化蛋白质组分析显示,杂合基因敲入小鼠突触前和突触后主要蛋白质的磷酸化状态发生了改变。与此相一致,我们检测到基因敲入小鼠海马神经元突触成熟度降低,海马长期电位减弱。总之,杂合子基因敲入小鼠(CK2αK198R/+)表现出显著的表面有效性,呈现出与 ASD 相关的表型、突触缺陷和突触可塑性改变,所有这些都有力地验证了该品系作为 OCNDS 小鼠模型的有效性。本文章由计算机程序翻译,如有差异,请以英文原文为准。

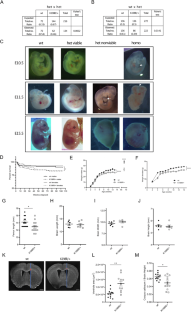
Missense mutation in the activation segment of the kinase CK2 models Okur-Chung neurodevelopmental disorder and alters the hippocampal glutamatergic synapse
Exome sequencing has enabled the identification of causative genes of monogenic forms of autism, amongst them, in 2016, CSNK2A1, the gene encoding the catalytic subunit of the kinase CK2, linking this kinase to Okur-Chung Neurodevelopmental Syndrome (OCNDS), a newly described neurodevelopmental condition with many symptoms resembling those of autism spectrum disorder. Thus far, no preclinical model of this condition exists. Here we describe a knock-in mouse model that harbors the K198R mutation in the activation segment of the α subunit of CK2. This region is a mutational hotspot, representing one-third of patients. These mice exhibit behavioral phenotypes that mirror patient symptoms. Homozygous knock-in mice die mid-gestation while heterozygous knock-in mice are born at half of the expected mendelian ratio and are smaller in weight and size than wildtype littermates. Heterozygous knock-in mice showed alterations in cognition and memory-assessing paradigms, enhanced stereotypies, altered circadian activity patterns, and nesting behavior. Phosphoproteome analysis from brain tissue revealed alterations in the phosphorylation status of major pre- and postsynaptic proteins of heterozygous knock-in mice. In congruence, we detect reduced synaptic maturation in hippocampal neurons and attenuated long-term potentiation in the hippocampus of knock-in mice. Taken together, heterozygous knock-in mice (CK2αK198R/+) exhibit significant face validity, presenting ASD-relevant phenotypes, synaptic deficits, and alterations in synaptic plasticity, all of which strongly validate this line as a mouse model of OCNDS.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Molecular Psychiatry
医学-精神病学
CiteScore
20.50
自引率
4.50%
发文量
459
审稿时长
4-8 weeks
期刊介绍:
Molecular Psychiatry focuses on publishing research that aims to uncover the biological mechanisms behind psychiatric disorders and their treatment. The journal emphasizes studies that bridge pre-clinical and clinical research, covering cellular, molecular, integrative, clinical, imaging, and psychopharmacology levels.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


