在表达 Trib1 的急性髓性白血病(AML)细胞中,Cop1 基因缺失导致 C/EBPα p42 快速增加,从而诱导急性髓性白血病(AML)细胞生长停滞
IF 12.8
1区 医学
Q1 HEMATOLOGY
引用次数: 0
摘要
Cop1 是一种泛素 E3 连接酶,在植物和类人猿的进化过程中都得到了很好的保存。在后生动物中,C/EBP 家族转录因子是 Cop1 的降解靶标,这一过程受 Tribbles 伪激酶家族的调控。过度表达Tribbles同源物1(Trib1)可通过Cop1依赖性降解C/EBPα p42异构体诱导急性髓性白血病(AML)。在这里,我们利用Cop1条件性敲除(KO)诱导表达Trib1的AML细胞快速生长停滞和粒细胞分化,这与C/EBPα p42同工酶的短暂增加有关。通过沉默 Cebpa 可以消除 Cop1 KO 的生长抑制作用,通过外源表达 p42 同工酶可以加强这种作用。此外,Cop1 KO 提高了移植了表达 Trib1 的 AML 细胞的受体的存活率。我们进一步发现,在 Cop1 KO 的情况下,Trib1 蛋白表达明显增加,这表明 Trib1 被 Cop1 降解体自我降解。下调 COP1 还能以 TRIB1 依赖性方式抑制人类 AML 细胞的增殖。综上所述,我们的研究结果为了解 Trib1/Cop1 机制在 C/EBPα p42 依赖性致白血病活性中的作用提供了新的视角,也为开发新的治疗方法提供了新的思路。本文章由计算机程序翻译,如有差异,请以英文原文为准。
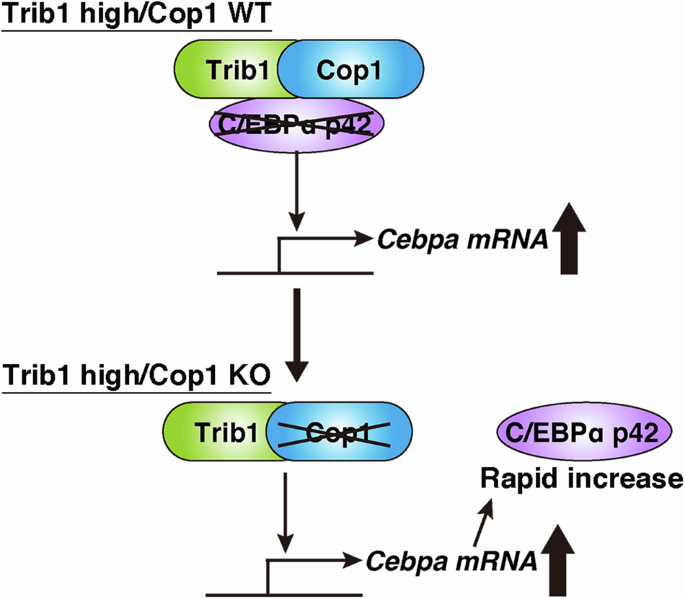
Rapid increase of C/EBPα p42 induces growth arrest of acute myeloid leukemia (AML) cells by Cop1 deletion in Trib1-expressing AML
Cop1 encodes a ubiquitin E3 ligase that has been well preserved during evolution in both plants and metazoans. In metazoans, the C/EBP family transcription factors are targets for degradation by Cop1, and this process is regulated by the Tribbles pseudokinase family. Over-expression of Tribbles homolog 1 (Trib1) induces acute myeloid leukemia (AML) via Cop1-dependent degradation of the C/EBPα p42 isoform. Here, we induced rapid growth arrest and granulocytic differentiation of Trib1-expressing AML cells using a Cop1 conditional knockout (KO), which is associated with a transient increase in the C/EBPα p42 isoform. The growth-suppressive effect of Cop1 KO was canceled by silencing of Cebpa and reinforced by exogenous expression of the p42 isoform. Moreover, Cop1 KO improved the survival of recipients transplanted with Trib1-expressing AML cells. We further identified a marked increase in Trib1 protein expression in Cop1 KO, indicating that Trib1 is self-degraded by the Cop1 degradosome. COP1 downregulation also inhibits the proliferation of human AML cells in a TRIB1-dependent manner. Taken together, our results provide new insights into the role of Trib1/Cop1 machinery in the C/EBPα p42-dependent leukemogenic activity, and a novel idea to develop new therapeutics.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Leukemia
医学-血液学
CiteScore
18.10
自引率
3.50%
发文量
270
审稿时长
3-6 weeks
期刊介绍:
Title: Leukemia
Journal Overview:
Publishes high-quality, peer-reviewed research
Covers all aspects of research and treatment of leukemia and allied diseases
Includes studies of normal hemopoiesis due to comparative relevance
Topics of Interest:
Oncogenes
Growth factors
Stem cells
Leukemia genomics
Cell cycle
Signal transduction
Molecular targets for therapy
And more
Content Types:
Original research articles
Reviews
Letters
Correspondence
Comments elaborating on significant advances and covering topical issues
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


