胶质瘤相关癫痫的分子机制和诊断模型。
IF 6.8
1区 医学
Q1 ONCOLOGY
引用次数: 0
摘要
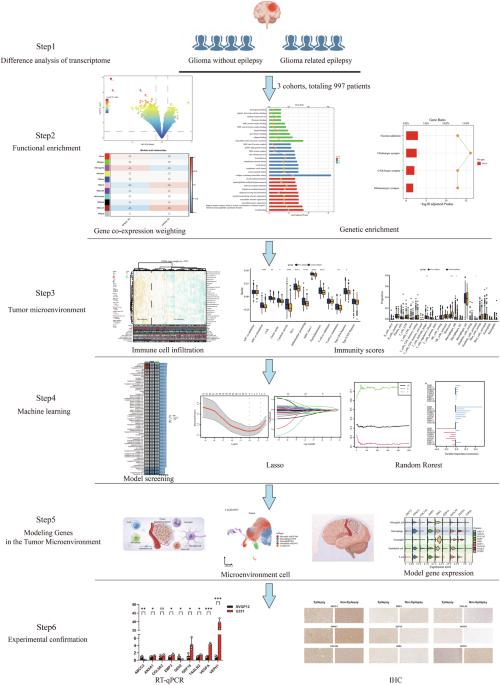
癫痫是胶质瘤患者最常见的症状之一,但其相互作用的机制尚不清楚。此外,流行病学研究并未准确识别出胶质瘤相关癫痫(GRE)患者,因此迫切需要确定其发生的分子机制和标记物。我们分析了997名胶质瘤患者的人口统计学、转录组、全基因组和甲基化序列,以确定胶质瘤和GRE患者的遗传差异,并确定上调的分子功能、细胞组成、参与的生物过程、信号通路和免疫细胞浸润。12种机器学习算法被细化为113种组合算法,用于建立诊断识别模型。共有 342 名 GRE 患者被确定为 WHO 2 级(174 人)、3 级(107 人)和 4 级(61 人)。GRE患者的平均年龄为38岁,其中有IDH突变(217人[63%])和1p19q非缺失(169人[49%])。GRE 分子功能主要是被动跨膜转运蛋白活性、离子通道活性和门控通道活性。细胞成分富含阳离子通道和跨膜转运体复合物。大脑皮层发育调节膜电位和突触组织是主要的生物学过程。信号通路主要集中在胆碱能、GABA能和谷氨酸能突触。LASSO与随机森林相结合是最佳诊断模型,并确定了9个诊断基因。这项研究为解决 GRE 的分子机制问题提供了新的见解和未来展望。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Molecular mechanisms and diagnostic model of glioma-related epilepsy
Epilepsy is one of the most common symptoms in patients with gliomas; however, the mechanisms underlying its interaction are not yet clear. Moreover, epidemiological studies have not accurately identified patients with glioma-related epilepsy (GRE), and there is an urgent need to identify the molecular mechanisms and markers of its occurrence. We analyzed the demographics, transcriptome, whole-genome, and methylation sequences of 997 patients with glioma, to determine the genetic differences between glioma and GRE patients and to determine the upregulated molecular function, cellular composition, biological processes involved, signaling pathways, and immune cell infiltration. Twelve machine learning algorithms were refined into 113 combinatorial algorithms for building diagnostic recognition models. A total of 342 patients with GRE were identified with WHO grade 2 (174), grade 3 (107), and grade 4 (61). The mean age of the patients with GREs, with IDH mutations (n = 217 [63%]) and 1p19q non-codeletion (n = 169 [49%]), was 38 years old. GRE molecular functions were mainly passive transmembrane transporter protein activity, ion channel activity, and gated channel activity. Cellular components were enriched in the cation-channel and transmembrane transporter complexes. Cerebral cortical development regulates the membrane potential and synaptic organization as major biological processes. The signaling pathways mainly focused on cholinergic, GABAergic, and glutamatergic synapses. LASSO, combined with Random Forest, was the best diagnostic model and identified nine diagnostic genes. This study provides new insights and future perspectives for resolving the molecular mechanisms of GRE.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

NPJ Precision Oncology
ONCOLOGY-
CiteScore
9.90
自引率
1.30%
发文量
87
审稿时长
18 weeks
期刊介绍:
Online-only and open access, npj Precision Oncology is an international, peer-reviewed journal dedicated to showcasing cutting-edge scientific research in all facets of precision oncology, spanning from fundamental science to translational applications and clinical medicine.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


