一种新型长非编码 RNA 将肥胖与脂肪细胞功能受损联系在一起。
IF 7
2区 医学
Q1 ENDOCRINOLOGY & METABOLISM
引用次数: 0
摘要
背景:长非编码 RNA(lncRNA)可在脂肪细胞中执行重要的相关任务,一旦出现缺陷,就会导致肥胖及其后遗症。在此,通过对减肥手术前后脂肪组织转录组(GSE53378)的仔细研究,确定了与肥胖表型相关的 496 个 lncRNA。只有linc-GALNTL6-4的表达量平均恢复了2倍以上,且经FDR调整后P值为方法:我们对公共数据集 GSE199063 进行了转录组分析,并在两个大型横向队列中进行了交叉验证,报告了之前未知的脂肪 linc-GALNTL6-4 与肥胖相关的证据。然后,我们在人类脂肪细胞培养物中进行了功能分析,在功能丧失和获得的细胞模型中进行了全基因组转录组学分析和非靶向脂质组学分析,以探索其与肥胖和体重减轻相关性的分子意义:结果:linc-GALNTL6-4在人类脂肪组织中的表达具有脂肪细胞特异性,并且与肥胖存在共分离现象,在体重减轻时表达趋于正常。两项纵向体重减轻研究和两项横断面样本都证实了这种共分离现象。在肥胖症患者中,linc-GALNTL6-4 的表达受到影响主要是由于肥胖症中的炎症成分,而脂肪生成需要 linc-GALNTL6-4 的转录上调,其表达在终末分化的脂肪细胞中达到顶峰。在功能上,我们证明了敲除 linc-GALNTL6-4 会损害脂肪的生成,诱导脂质体的改变,并导致细胞周期相关基因的下调,同时推动脂肪细胞中的炎症、脂肪酸代谢受损和基因表达模式的改变,包括脂蛋白 C1(APOC1)的表达模式。相反,linc-GALNTL6-4的基因增殖改善了分化和脂肪细胞表型,这可能是通过限制APOC1实现的,也有助于脂肪中甘油三酯的代谢:目前的数据揭示了脂肪细胞特异性 linc-GALNTL6-4 作为受体重过重和元炎症挑战的脂质稳态调节器的不可预见的联系。本文章由计算机程序翻译,如有差异,请以英文原文为准。
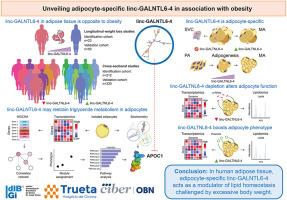
A novel long non-coding RNA connects obesity to impaired adipocyte function
Background
Long non-coding RNAs (lncRNAs) can perform tasks of key relevance in fat cells, contributing, when defective, to the burden of obesity and its sequelae. Here, scrutiny of adipose tissue transcriptomes before and after bariatric surgery (GSE53378) granted identification of 496 lncRNAs linked to the obese phenotype. Only expression of linc-GALNTL6-4 displayed an average recovery over 2-fold and FDR-adjusted p-value <0.0001 after weight loss. The aim of the present study was to investigate the impact on adipocyte function and potential clinical value of impaired adipose linc-GALNTL6-4 in obese subjects.
Methods
We employed transcriptomic analysis of public dataset GSE199063, and cross validations in two large transversal cohorts to report evidence of a previously unknown association of adipose linc-GALNTL6-4 with obesity. We then performed functional analyses in human adipocyte cultures, genome-wide transcriptomics, and untargeted lipidomics in cell models of loss and gain of function to explore the molecular implications of its associations with obesity and weight loss.
Results
The expression of linc-GALNTL6-4 in human adipose tissue is adipocyte-specific and co-segregates with obesity, being normalized upon weight loss. This co-segregation is demonstrated in two longitudinal weight loss studies and two cross-sectional samples. While compromised expression of linc-GALNTL6-4 in obese subjects is primarily due to the inflammatory component in the context of obesity, adipogenesis requires the transcriptional upregulation of linc-GALNTL6-4, the expression of which reaches an apex in terminally differentiated adipocytes. Functionally, we demonstrated that the knockdown of linc-GALNTL6-4 impairs adipogenesis, induces alterations in the lipidome, and leads to the downregulation of genes related to cell cycle, while propelling in adipocytes inflammation, impaired fatty acid metabolism, and altered gene expression patterns, including that of apolipoprotein C1 (APOC1). Conversely, the genetic gain of linc-GALNTL6-4 ameliorated differentiation and adipocyte phenotype, putatively by constraining APOC1, also contributing to the metabolism of triglycerides in adipose.
Conclusions
Current data unveil the unforeseen connection of adipocyte-specific linc-GALNTL6-4 as a modulator of lipid homeostasis challenged by excessive body weight and meta-inflammation.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Molecular Metabolism
ENDOCRINOLOGY & METABOLISM-
CiteScore
14.50
自引率
2.50%
发文量
219
审稿时长
43 days
期刊介绍:
Molecular Metabolism is a leading journal dedicated to sharing groundbreaking discoveries in the field of energy homeostasis and the underlying factors of metabolic disorders. These disorders include obesity, diabetes, cardiovascular disease, and cancer. Our journal focuses on publishing research driven by hypotheses and conducted to the highest standards, aiming to provide a mechanistic understanding of energy homeostasis-related behavior, physiology, and dysfunction.
We promote interdisciplinary science, covering a broad range of approaches from molecules to humans throughout the lifespan. Our goal is to contribute to transformative research in metabolism, which has the potential to revolutionize the field. By enabling progress in the prognosis, prevention, and ultimately the cure of metabolic disorders and their long-term complications, our journal seeks to better the future of health and well-being.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


