PCSK9抑制剂通过抑制炎症反应和TGF-β1/Smad3通路减轻再灌注损伤大鼠的心脏纤维化
IF 5.3
2区 医学
Q1 PHARMACOLOGY & PHARMACY
引用次数: 0
摘要
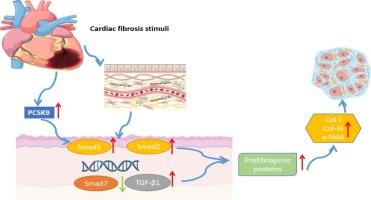
进行性心脏纤维化是心力衰竭的标志之一,但人们对PCSK9(Protein convertase subtilisin/kexin type 9)的作用仍然知之甚少。本研究旨在阐明PCSK9在心脏纤维化中的作用。在对大鼠进行缺血再灌注(I/R)损伤手术后,使用PCSK9抑制剂检测其对转化生长因子-β1(TGF-β1)/小母抗癸型截瘫因子3(Smad3)通路和炎症的影响。在I/R损伤后的心脏组织中观察到PCSK9、TGF-β1和Smad3水平升高,表明心脏纤维化。抑制 PCSK9 可减少促纤维化蛋白的表达,从而保护心脏,减轻 I/R 引起的损伤和纤维化。此外,它还能改善心脏炎症,缩小心肌梗死(MI)后的面积,改善心脏功能,减缓心力衰竭的进展。PCSK9抑制剂能明显减轻I/R通过TGF-β1/Smad3途径诱导的心肌纤维化。本文章由计算机程序翻译,如有差异,请以英文原文为准。
PCSK9 inhibitor attenuates cardiac fibrosis in reperfusion injury rat by suppressing inflammatory response and TGF-β1/Smad3 pathway
Progressive cardiac fibrosis, a hallmark of heart failure, remains poorly understood regarding Proprotein convertase subtilisin/kexin type 9 (PCSK9) ’s role. This study aims to elucidate PCSK9′s involvement in cardiac fibrosis. After ischemia/reperfusion (I/R) injury surgery in rats, PCSK9 inhibitors were used to examine their effects on the transforming growth factor-β1 (TGF-β1)/small mother against decapentaplegic 3 (Smad3) pathway and inflammation. Elevated PCSK9, TGF-β1, and Smad3 levels were observed in cardiac tissues post-I/R injury, indicating fibrosis. PCSK9 inhibition reduced pro-fibrotic protein expression, protecting the heart and mitigating I/R-induced damage and fibrosis. Additionally, it ameliorated cardiac inflammation and reduced post-myocardial infarction (MI) size, improving cardiac function and slowing heart failure progression. PCSK9 inhibitors significantly attenuate myocardial fibrosis induced by I/R via the TGF-β1/Smad3 pathway.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Biochemical pharmacology
医学-药学
CiteScore
10.30
自引率
1.70%
发文量
420
审稿时长
17 days
期刊介绍:
Biochemical Pharmacology publishes original research findings, Commentaries and review articles related to the elucidation of cellular and tissue function(s) at the biochemical and molecular levels, the modification of cellular phenotype(s) by genetic, transcriptional/translational or drug/compound-induced modifications, as well as the pharmacodynamics and pharmacokinetics of xenobiotics and drugs, the latter including both small molecules and biologics.
The journal''s target audience includes scientists engaged in the identification and study of the mechanisms of action of xenobiotics, biologics and drugs and in the drug discovery and development process.
All areas of cellular biology and cellular, tissue/organ and whole animal pharmacology fall within the scope of the journal. Drug classes covered include anti-infectives, anti-inflammatory agents, chemotherapeutics, cardiovascular, endocrinological, immunological, metabolic, neurological and psychiatric drugs, as well as research on drug metabolism and kinetics. While medicinal chemistry is a topic of complimentary interest, manuscripts in this area must contain sufficient biological data to characterize pharmacologically the compounds reported. Submissions describing work focused predominately on chemical synthesis and molecular modeling will not be considered for review.
While particular emphasis is placed on reporting the results of molecular and biochemical studies, research involving the use of tissue and animal models of human pathophysiology and toxicology is of interest to the extent that it helps define drug mechanisms of action, safety and efficacy.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


