Midkine 是乳腺肿瘤发生中与年龄有关的变化和增加的驱动因素
IF 44.5
1区 医学
Q1 CELL BIOLOGY
引用次数: 0
摘要
衰老是癌症的一个关键风险因素,但其潜在机制仍未得到很好的界定。在这里,我们通过单细胞多组学研究探讨了大鼠乳腺中与年龄相关的变化。我们的发现包括随着年龄的增长,上皮细胞增殖增加、管腔特性丧失、幼稚 B 细胞和 T 细胞减少。我们发现了老龄大鼠特有的管腔祖细胞群,其特征反映了癌前病变,并确定 Midkine(Mdk)是随年龄增长而上调的基因,也是与年龄相关的管腔祖细胞的调节因子。通过激活 PI3K-AKT-SREBF1 通路,对年轻大鼠进行 Midkine 处理可模拟与年龄相关的变化,并促进亚硝基-N-甲基脲诱导的乳腺肿瘤发生。人体血液和乳腺上皮细胞中的 Midkine 水平会随着年龄的增长而增加,正常乳腺组织中较高的 MDK 与年轻女性较高的乳腺癌风险有关。我们的研究结果揭示了衰老与肿瘤发生易感性之间的联系,并确定 Midkine 是乳腺肿瘤发生随年龄增长而增加的介质。本文章由计算机程序翻译,如有差异,请以英文原文为准。
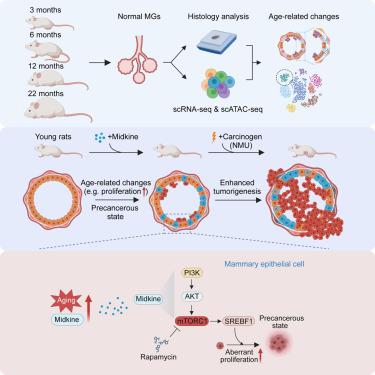
Midkine as a driver of age-related changes and increase in mammary tumorigenesis
Aging is a pivotal risk factor for cancer, yet the underlying mechanisms remain poorly defined. Here, we explore age-related changes in the rat mammary gland by single-cell multiomics. Our findings include increased epithelial proliferation, loss of luminal identity, and decreased naive B and T cells with age. We discover a luminal progenitor population unique to old rats with profiles reflecting precancerous changes and identify midkine (Mdk) as a gene upregulated with age and a regulator of age-related luminal progenitors. Midkine treatment of young rats mimics age-related changes via activating PI3K-AKT-SREBF1 pathway and promotes nitroso-N-methylurea-induced mammary tumorigenesis. Midkine levels increase with age in human blood and mammary epithelium, and higher MDK in normal breast tissue is associated with higher breast cancer risk in younger women. Our findings reveal a link between aging and susceptibility to tumor initiation and identify midkine as a mediator of age-dependent increase in breast tumorigenesis.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Cancer Cell
医学-肿瘤学
CiteScore
55.20
自引率
1.20%
发文量
179
审稿时长
4-8 weeks
期刊介绍:
Cancer Cell is a journal that focuses on promoting major advances in cancer research and oncology. The primary criteria for considering manuscripts are as follows:
Major advances: Manuscripts should provide significant advancements in answering important questions related to naturally occurring cancers.
Translational research: The journal welcomes translational research, which involves the application of basic scientific findings to human health and clinical practice.
Clinical investigations: Cancer Cell is interested in publishing clinical investigations that contribute to establishing new paradigms in the treatment, diagnosis, or prevention of cancers.
Insights into cancer biology: The journal values clinical investigations that provide important insights into cancer biology beyond what has been revealed by preclinical studies.
Mechanism-based proof-of-principle studies: Cancer Cell encourages the publication of mechanism-based proof-of-principle clinical studies, which demonstrate the feasibility of a specific therapeutic approach or diagnostic test.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


