短叶皂甙 C 对巨噬细胞衍生的衣康酸的药理上调减轻了心肌缺血再灌注损伤。
IF 13
1区 综合性期刊
Q1 MULTIDISCIPLINARY SCIENCES
引用次数: 0
摘要
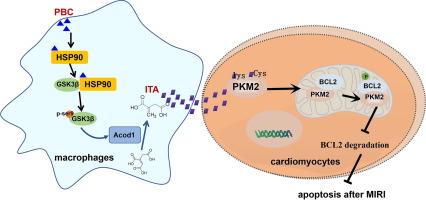
简介心肌缺血再灌注损伤(MIRI)仍然是全球普遍存在的临床难题,缺乏理想的治疗策略。巨噬细胞在心肌缺血再灌注损伤的病理生理学中发挥着关键作用,具有动态的炎症和溶解功能。巨噬细胞的极化和新陈代谢与 MIRI 密切相关,是潜在的治疗靶点。从 Ilex pubescens 中提取的阳起石甙 C(PBC)具有明显的抗炎作用,但 PBC 对 MIRI 的影响尚不清楚:本研究旨在评估 PBC 对 MIRI 的心脏保护作用,并阐明其潜在机制:方法:使用 Sprague-Dawley 大鼠、H9c2 和 RAW264.7 巨噬细胞建立 MIRI 的体外和体内模型。采用TTC/Evans蓝染色、免疫组织化学染色、代谢组学分析、化学探针、表面等离子体共振(SPR)、共免疫沉淀(CO-IP)等方法进行药效学和机制研究:结果:PBC能有效缩小心肌梗死面积、降低ST段抬高、降低CK-MB水平,同时促进MIRI中巨噬细胞M2极化。此外,经 PBC 处理的巨噬细胞及其条件培养液可减轻氧-葡萄糖剥夺/复氧(OGD/R)诱导的 H9c2 细胞凋亡。代谢组学分析表明,PBC 增加了巨噬细胞中衣康酸(ITA)和苹果酸(MA)的产生,从而保护 H9c2 细胞免受 OGD/R 损伤。机理研究表明,ITA是通过共价修饰丙酮酸激酶M2(PKM2)的Cys474、Cys424和Lys151,从而促进PKM2的线粒体转位并增强PKM2/Bcl2的相互作用,进而导致Bcl2降解减少来发挥其作用的。SPR 分析进一步显示,PBC 与 HSP90 结合,促进了 HSP90 与 GSK3β 之间的相互作用,导致 GSK3β 活性失活,并上调了产生 ITA 和 MA 的关键代谢酶(Acod1 和 Mdh2):结论:PBC 可通过调节 HSP90/ITA/PKM2 轴缓解 MIRI 诱导的心肌细胞凋亡。结论:PBC 通过调节 HSP90/ITA/PKM2 轴,减轻了 MIRI 诱导的心肌细胞凋亡。此外,药理学上调 ITA 是治疗 MIRI 的一种很有前景的方法,这表明 PBC 有可能成为治疗 MIRI 的候选药物。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Pharmacological upregulation of macrophage-derived itaconic acid by pubescenoside C attenuated myocardial ischemia–reperfusion injury
Introduction
Myocardial ischemia–reperfusion injury (MIRI) remains a prevalent clinical challenge globally, lacking an ideal therapeutic strategy. Macrophages play a pivotal role in MIRI pathophysiology, exhibiting dynamic inflammatory and resolutive functions. Macrophage polarization and metabolism are intricately linked to MIRI, presenting potential therapeutic targets. Pubescenoside C (PBC) from Ilex pubescens showed significantly anti-inflammatory effects, however, the effect of PBC on MIRI is unknown.
Objectives
This study aimed to assess the cardioprotective effects of PBC against MIRI and elucidate the underlying mechanisms.
Methods
Sprague-Dawley rats, H9c2 and RAW264.7 macrophages were used to establish the in vitro and in vivo models of MIRI. TTC/Evans blue staining, immunohistochemical staining, metabonomics analysis, chemical probe, surface plasmon resonance (SPR), co-immunoprecipitation (CO-IP) assays were used for pharmacodynamic and mechanism study.
Results
PBC administration effectively reduced myocardial infarct size, decreased ST-segment elevation, and lowered CK-MB levels, concurrently promoting macrophage M2 polarization in MIRI. Furthermore, PBC-treated macrophages and their conditioned culture medium attenuated the apoptosis of H9c2 cells induced by oxygen-glucose deprivation/reoxygenation (OGD/R). Metabonomics analysis revealed that PBC increased the production of itaconic acid (ITA) and malic acid (MA) in macrophages, which conferred protection against OGD/R injury in H9c2 cells. Mechanistic investigations indicated that ITA exerted its effects by covalently modifying pyruvate kinase M2 (PKM2) at Cys474, Cys424, and Lys151, thereby facilitating PKM2′s mitochondrial translocation and enhancing the PKM2/Bcl2 interaction, subsequently leading to decreased degradation of Bcl2. SPR assays further revealed that PBC bound to HSP90, facilitating the interaction between HSP90 and GSK3β and resulting in the inactivation of GSK3β activity and upregulation of key metabolic enzymes for ITA and MA production (Acod1 and Mdh2).
Conclusion
PBC alleviates MIRI-induced cardiomyocyte apoptosis by modulating the HSP90/ITA/PKM2 axis. Furthermore, pharmacological upregulation of ITA emerges as a promising therapeutic approach for MIRI, hinting at PBC’s potential as a candidate drug for MIRI therapy.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Advanced Research
Multidisciplinary-Multidisciplinary
CiteScore
21.60
自引率
0.90%
发文量
280
审稿时长
12 weeks
期刊介绍:
Journal of Advanced Research (J. Adv. Res.) is an applied/natural sciences, peer-reviewed journal that focuses on interdisciplinary research. The journal aims to contribute to applied research and knowledge worldwide through the publication of original and high-quality research articles in the fields of Medicine, Pharmaceutical Sciences, Dentistry, Physical Therapy, Veterinary Medicine, and Basic and Biological Sciences.
The following abstracting and indexing services cover the Journal of Advanced Research: PubMed/Medline, Essential Science Indicators, Web of Science, Scopus, PubMed Central, PubMed, Science Citation Index Expanded, Directory of Open Access Journals (DOAJ), and INSPEC.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


