在帕金森大鼠模型中,生物反应器产生的iPSCs衍生的含多巴胺能神经元的神经微组织以剂量依赖的方式支配旋转偏差并使其恢复正常。
IF 5.6
2区 医学
Q1 CLINICAL NEUROLOGY
引用次数: 0
摘要
目前,大量临床前研究支持多能干细胞衍生细胞替代疗法缓解帕金森患者运动症状的理论依据。这些疗法的主要重点是替代疾病中的主要功能障碍细胞群,即A9多巴胺能神经元。为了实现这一目标,大多数治疗方法都涉及移植单细胞悬浮的多巴胺能祖细胞。然而,大多数细胞在移植过程中死亡,因为细胞面临无免疫反应。应对这一挑战的一个潜在解决方案是移植固体制剂,即采用三维格式。冷冻保存这种形式的细胞仍然是一个主要障碍,而且不排除会导致起效时间延迟,正如在冷冻保存的单细胞DA祖细胞中观察到的那样。在这里,我们利用高通量细胞封存技术和生物反应器提供了一个三维培养环境,使 hiPSCs 能够定向分化成神经微组织。通过qPCR、RNAseq、流式细胞术和免疫荧光显微镜等正交方法证实了这些神经微组织的中脑特征。神经微组织的功效通过帕金森大鼠模型以剂量依赖的方式得到了证实。死后组织学分析证实了细胞的存活,其特征是存在投射到宿主纹状体的人类多巴胺能神经元。本文报告的工作是首次在可扩展的生物反应器中对帕金森病进行细胞治疗的生物生产,利用低温保存的三维格式在移植 16 周后实现了完全的行为恢复。本文章由计算机程序翻译,如有差异,请以英文原文为准。
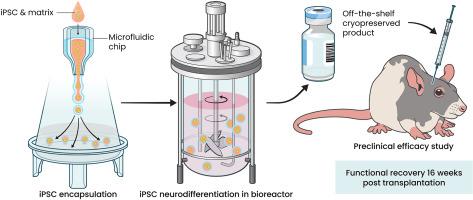
Bioreactor-produced iPSCs-derived dopaminergic neuron-containing neural microtissues innervate and normalize rotational bias in a dose-dependent manner in a Parkinson rat model
A breadth of preclinical studies now support the rationale of pluripotent stem cell-derived cell replacement therapies to alleviate motor symptoms in Parkinsonian patients. Replacement of the primary dysfunctional cell population in the disease, i.e. the A9 dopaminergic neurons, is the major focus of these therapies. To achieve this, most therapeutical approaches involve grafting single-cell suspensions of DA progenitors. However, most cells die during the transplantation process, as cells face anoïkis. One potential solution to address this challenge is to graft solid preparations, i.e. adopting a 3D format. Cryopreserving such a format remains a major hurdle and is not exempt from causing delays in the time to effect, as observed with cryopreserved single-cell DA progenitors. Here, we used a high-throughput cell-encapsulation technology coupled with bioreactors to provide a 3D culture environment enabling the directed differentiation of hiPSCs into neural microtissues. The proper patterning of these neural microtissues into a midbrain identity was confirmed using orthogonal methods, including qPCR, RNAseq, flow cytometry and immunofluorescent microscopy. The efficacy of the neural microtissues was demonstrated in a dose-dependent manner using a Parkinsonian rat model. The survival of the cells was confirmed by post-mortem histological analysis, characterised by the presence of human dopaminergic neurons projecting into the host striatum. The work reported here is the first bioproduction of a cell therapy for Parkinson's disease in a scalable bioreactor, leading to a full behavioural recovery 16 weeks after transplantation using cryopreserved 3D format.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Neurotherapeutics
医学-神经科学
CiteScore
11.00
自引率
3.50%
发文量
154
审稿时长
6-12 weeks
期刊介绍:
Neurotherapeutics® is the journal of the American Society for Experimental Neurotherapeutics (ASENT). Each issue provides critical reviews of an important topic relating to the treatment of neurological disorders written by international authorities.
The Journal also publishes original research articles in translational neuroscience including descriptions of cutting edge therapies that cross disciplinary lines and represent important contributions to neurotherapeutics for medical practitioners and other researchers in the field.
Neurotherapeutics ® delivers a multidisciplinary perspective on the frontiers of translational neuroscience, provides perspectives on current research and practice, and covers social and ethical as well as scientific issues.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


