长非编码 RNA UCA1 通过激活 PPARα 介导的脂质代谢抑制表柔比星诱导的细胞凋亡
IF 3.5
3区 生物学
Q3 CELL BIOLOGY
引用次数: 0
摘要
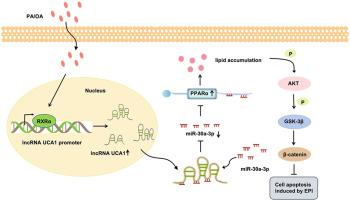
代谢重编程是癌症的一个标志,而脂质代谢异常与膀胱癌细胞的耐药性有关。长非编码 RNA(lncRNA)UCA1 在膀胱癌中过度表达,但其对脂质代谢的功能贡献仍未定性。在这项研究中,我们证实了lncRNA UCA1通过支持膀胱癌细胞中异常的脂质代谢来抑制表柔比星诱导的细胞凋亡。从机理上讲,lncRNA UCA1通过上调PPARα mRNA和蛋白的表达来促进体外和体内的脂质积累,而这是由miR-30a-3p介导的。敲除lncRNA UCA1可通过miR-30a-3p/PPARα和下游p-AKT/p-GSK-3β/β-catenin信号转导增加表柔比星诱导的细胞凋亡。此外,混合游离脂肪酸通过促进转录因子RXRα招募到lncRNA UCA1启动子,从而上调lncRNA UCA1的表达。这些发现在小鼠异种移植模型中得到了验证,并且与人类膀胱癌患者的表达模式一致。总之,这些发现确定了lncRNA UCA1在脂质代谢和膀胱癌细胞对表柔比星的耐药性中的作用,表明lncRNA UCA1可作为加强膀胱癌化疗的候选靶点。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Long noncoding RNA UCA1 inhibits epirubicin-induced apoptosis by activating PPARα-mediated lipid metabolism
Metabolic reprogramming is a hallmark of cancer, and abnormal lipid metabolism is associated with drug resistance in bladder cancer cells. The long noncoding RNA (lncRNA) UCA1 is overexpressed in bladder cancer, but its functional contribution to lipid metabolism remains uncharacterized. In this study, we demonstrated that lncRNA UCA1 inhibits epirubicin-induced cell apoptosis by supporting abnormal lipid metabolism in bladder cancer cells. Mechanistically, lncRNA UCA1 promotes lipid accumulation in vitro and in vivo by upregulating PPARα mRNA and protein expression, which is mediated by miR-30a-3p. Knockdown of lncRNA UCA1 increased epirubicin-induced apoptosis via miR-30a-3p/PPARα and downstream p-AKT/p-GSK-3β/β-catenin signaling. Furthermore, mixed free fatty acids upregulated lncRNA UCA1 expression by promoting recruitment of the transcription factor RXRα to the lncRNA UCA1 promoter. These findings were verified in a mouse xenograft model and are consistent with the expression patterns in human bladder cancer patients. Overall, these findings establish the role of lncRNA UCA1 in lipid metabolism and bladder cancer cell resistance to epirubicin, suggesting that lncRNA UCA1 may serve as a candidate target for enhancing bladder cancer chemotherapy.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Experimental cell research
医学-细胞生物学
CiteScore
7.20
自引率
0.00%
发文量
295
审稿时长
30 days
期刊介绍:
Our scope includes but is not limited to areas such as: Chromosome biology; Chromatin and epigenetics; DNA repair; Gene regulation; Nuclear import-export; RNA processing; Non-coding RNAs; Organelle biology; The cytoskeleton; Intracellular trafficking; Cell-cell and cell-matrix interactions; Cell motility and migration; Cell proliferation; Cellular differentiation; Signal transduction; Programmed cell death.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


