靶向 CREB 结合蛋白(CBP)可通过对 C-MYC 的表观遗传调控来削弱结直肠癌干性。
IF 5
3区 医学
Q1 BIOTECHNOLOGY & APPLIED MICROBIOLOGY
引用次数: 0
摘要
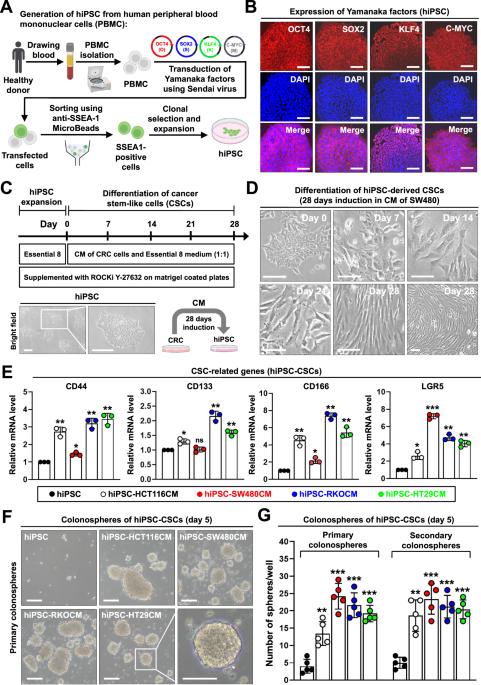
结直肠癌(CRC)是全球常见的癌症,发病率逐年上升。癌症干细胞(CSCs)在 CRC 的发生、发展、复发和转移中发挥着重要作用。调控结直肠癌干细胞发展的分子机制仍不清楚。通过体细胞重编程发现的人类诱导多能干细胞(hiPSCs)彻底改变了干细胞生物学和转化医学领域。在本研究中,我们用来自 CRC 细胞的条件培养基(CM)将 hiPSCs 转化为癌症干样细胞。这些转化细胞被称为hiPSC-CSCs,具有癌症干样特性,包括球形形态以及多能性和CSC标志物的表达。HiPSC-CSCs 在小鼠模型中表现出致癌和转移能力。在 hiPSC-CSCs 中观察到了上皮-间质转化表型,这促进了它们的迁移和血管生成。有趣的是,在 hiPSC-CSCs 分化过程中观察到了 C-MYC 的上调。从机制上讲,CREB结合蛋白(CBP)与C-MYC启动子结合,而组蛋白去乙酰化酶1和3(HDAC1/3)与启动子分离,最终导致组蛋白乙酰化增加和C-MYC转录激活。用CBP抑制剂进行药理处理或用基于CRISPR/Cas9的策略消减CBP的表达会降低hiPSC-造血干细胞的干性。这项研究首次证明,结直肠干细胞可由hiPSCs产生。在转化过程中,通过组蛋白乙酰化上调C-MYC起着至关重要的作用。抑制CBP是减弱结直肠造血干细胞干性的一种潜在策略。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Targeting CREB-binding protein (CBP) abrogates colorectal cancer stemness through epigenetic regulation of C-MYC
Colorectal cancer (CRC) is a common cancer worldwide with an increasing annual incidence. Cancer stem cells (CSCs) play important roles in the occurrence, development, recurrence, and metastasis of CRC. The molecular mechanism regulating the development of colorectal CSCs remains unclear. The discovery of human induced pluripotent stem cells (hiPSCs) through somatic cell reprogramming has revolutionized the fields of stem cell biology and translational medicine. In the present study, we converted hiPSCs into cancer stem-like cells by culture with conditioned medium (CM) from CRC cells. These transformed cells, termed hiPSC-CSCs, displayed cancer stem-like properties, including a spheroid morphology and the expression of both pluripotency and CSC markers. HiPSC-CSCs showed tumorigenic and metastatic abilities in mouse models. The epithelial-mesenchymal transition phenotype was observed in hiPSC-CSCs, which promoted their migration and angiogenesis. Interestingly, upregulation of C-MYC was observed during the differentiation of hiPSC-CSCs. Mechanistically, CREB binding protein (CBP) bound to the C-MYC promoter, while histone deacetylase 1 and 3 (HDAC1/3) dissociated from the promoter, ultimately leading to an increase in histone acetylation and C-MYC transcriptional activation during the differentiation of hiPSC-CSCs. Pharmacological treatment with a CBP inhibitor or abrogation of CBP expression with a CRISPR/Cas9-based strategy reduced the stemness of hiPSC-CSCs. This study demonstrates for the first time that colorectal CSCs can be generated from hiPSCs. The upregulation of C-MYC via histone acetylation plays a crucial role during the conversion process. Inhibition of CBP is a potential strategy for attenuating the stemness of colorectal CSCs.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Cancer gene therapy
医学-生物工程与应用微生物
CiteScore
10.20
自引率
0.00%
发文量
150
审稿时长
4-8 weeks
期刊介绍:
Cancer Gene Therapy is the essential gene and cellular therapy resource for cancer researchers and clinicians, keeping readers up to date with the latest developments in gene and cellular therapies for cancer. The journal publishes original laboratory and clinical research papers, case reports and review articles. Publication topics include RNAi approaches, drug resistance, hematopoietic progenitor cell gene transfer, cancer stem cells, cellular therapies, homologous recombination, ribozyme technology, antisense technology, tumor immunotherapy and tumor suppressors, translational research, cancer therapy, gene delivery systems (viral and non-viral), anti-gene therapy (antisense, siRNA & ribozymes), apoptosis; mechanisms and therapies, vaccine development, immunology and immunotherapy, DNA synthesis and repair.
Cancer Gene Therapy publishes the results of laboratory investigations, preclinical studies, and clinical trials in the field of gene transfer/gene therapy and cellular therapies as applied to cancer research. Types of articles published include original research articles; case reports; brief communications; review articles in the main fields of drug resistance/sensitivity, gene therapy, cellular therapy, tumor suppressor and anti-oncogene therapy, cytokine/tumor immunotherapy, etc.; industry perspectives; and letters to the editor.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


