阴离子响应螺旋 PtII 复合物的离子配对组装
IF 4.6
1区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
摘要
研究人员合成了萘基异构喹啉添加的二吡咯烷酮铂Ⅱ配合物螺旋π电子系统。这些铂Ⅱ配合物在溶液中显示出旋光特性,并表现出阴离子结合行为,从而形成了作为伪π电子阴离子的阴离子配合物。在与π-电子阳离子结合时,受体-阴离子配合物形成了逐电荷离子配对组合,在晶体状态下,自恋式自排序柱状结构由两种对映体中的任何一种组成。本文章由计算机程序翻译,如有差异,请以英文原文为准。
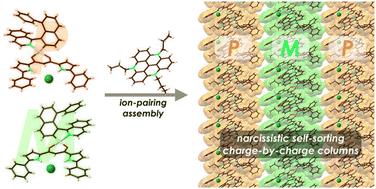
Ion-pairing assemblies of anion-responsive helical PtII complexes†
Naphthylisoquinoline-appended dipyrrolyldiketone PtII complexes as helical π-electronic systems were synthesized. The PtII complexes, showing chiroptical properties in solution, exhibited anion-binding behaviour, resulting in the formation of anion complexes as pseudo π-electronic anions. In combination with the π-electronic cation, the receptor–anion complexes formed charge-by-charge ion-pairing assemblies, with the narcissistic self-sorting columnar structures comprising either of the enantiomers, in the crystal state.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Organic Chemistry Frontiers
CHEMISTRY, ORGANIC-
CiteScore
7.90
自引率
11.10%
发文量
686
审稿时长
1 months
期刊介绍:
Organic Chemistry Frontiers is an esteemed journal that publishes high-quality research across the field of organic chemistry. It places a significant emphasis on studies that contribute substantially to the field by introducing new or significantly improved protocols and methodologies. The journal covers a wide array of topics which include, but are not limited to, organic synthesis, the development of synthetic methodologies, catalysis, natural products, functional organic materials, supramolecular and macromolecular chemistry, as well as physical and computational organic chemistry.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


