"通过相互学习协调欧盟数字医疗设备的评估和报销"。
IF 12.4
1区 医学
Q1 HEALTH CARE SCIENCES & SERVICES
引用次数: 0
摘要
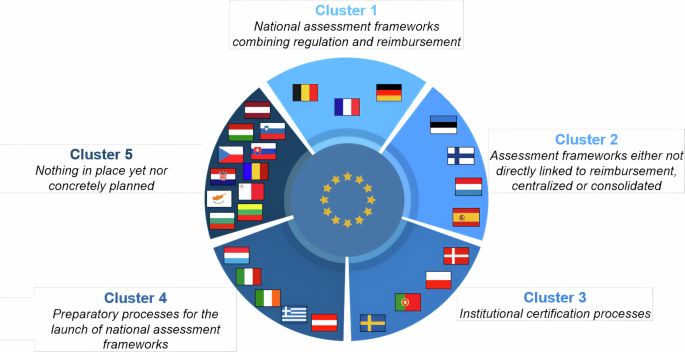
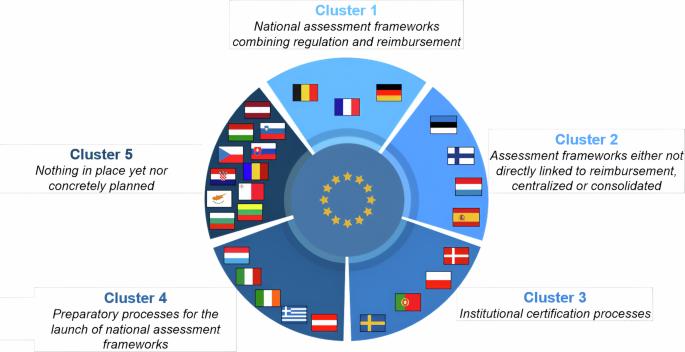
数字医疗设备 (DMD) 为其监管和报销带来了独特的机遇。欧盟内部正在形成一个动态的数字医疗设备评估框架,并确定了五组流行方法。尽管在成熟度上存在明显差距,但跨国学习效应正在变得普遍。我们期待欧盟内外有更多的国家效仿目前的先行者,从而加快协调进程。本文章由计算机程序翻译,如有差异,请以英文原文为准。
“Towards harmonizing assessment and reimbursement of digital medical devices in the EU through mutual learning”
Digital medical devices (DMDs) present unique opportunities in their regulation and reimbursement. A dynamic landscape of DMD assessment frameworks is emerging within the European Union, with five clusters of prevailing approaches identified. Despite notable gaps in maturity levels, cross-country learning effects are becoming prevalent. We expect more countries, both within the EU and beyond, to follow the steps of current frontrunners, hence expediting the harmonization process.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

NPJ Digital Medicine
Multiple-
CiteScore
25.10
自引率
3.30%
发文量
170
审稿时长
15 weeks
期刊介绍:
npj Digital Medicine is an online open-access journal that focuses on publishing peer-reviewed research in the field of digital medicine. The journal covers various aspects of digital medicine, including the application and implementation of digital and mobile technologies in clinical settings, virtual healthcare, and the use of artificial intelligence and informatics.
The primary goal of the journal is to support innovation and the advancement of healthcare through the integration of new digital and mobile technologies. When determining if a manuscript is suitable for publication, the journal considers four important criteria: novelty, clinical relevance, scientific rigor, and digital innovation.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


