通过碱促进的烯丙基化和逆迈克尔乙烯基脱氢亚硫酰化统一合成缺电子的 1,4- 和 1,1,4- 取代的 1,3- 二烯。
IF 4.9
1区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
摘要
我们揭示了一种分两步合成在共轭体系的两个末端均含有电子吸收取代基的贫电子 1,4- 和 1,1,4- 取代丁-1,3-二烯的方法。该方法依赖于伯或仲烷基砜与γ-溴巴豆酸盐或相关酰胺、腈或砜的碱促进 C-烯丙基化反应,以及随后的乙烯基逆迈克尔脱水亚磺酰化反应。生成的二烯的几何形状取决于底物,主要是由 E 型亲电体生成 E,E-二烯。这种不含磷和过渡金属的方法可容忍多种官能团,可作为 Wittig、HWE 和 Julia 烯化反应的补充。本文章由计算机程序翻译,如有差异,请以英文原文为准。
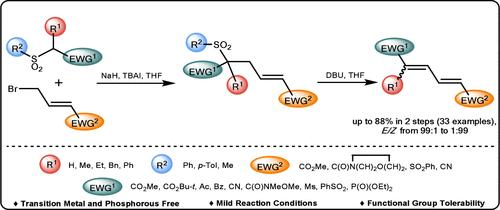
A Unified Synthesis of Electron-Deficient 1,4- and 1,1,4-Substituted 1,3-Dienes through a Base-Promoted Allylation Followed by Retro-Michael Vinylogous Dehydrosulfinylation.
We disclose a general 2-step synthesis of electron-poor 1,4- and 1,1,4-substituted buta-1,3-dienes bearing electron-withdrawing substituents at both termini of the conjugated system. The method relies on a base-promoted C-allylation of primary or secondary alkylsulfones with γ-bromocrotonate or related amide, nitrile, or sulfone and subsequent vinylogous retro-Michael dehydrosulfinylation. The geometry of the resulting dienes is substrate-dependent, and predominantly E,E-dienes are formed from E-electrophiles. This phosphorus- and transition-metal-free method tolerates a variety of functionalities and could serve as a supplement to Wittig, HWE, and Julia olefinations.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Organic Letters
化学-有机化学
CiteScore
9.30
自引率
11.50%
发文量
1607
审稿时长
1.5 months
期刊介绍:
Organic Letters invites original reports of fundamental research in all branches of the theory and practice of organic, physical organic, organometallic,medicinal, and bioorganic chemistry. Organic Letters provides rapid disclosure of the key elements of significant studies that are of interest to a large portion of the organic community. In selecting manuscripts for publication, the Editors place emphasis on the originality, quality and wide interest of the work. Authors should provide enough background information to place the new disclosure in context and to justify the rapid publication format. Back-to-back Letters will be considered. Full details should be reserved for an Article, which should appear in due course.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


