甲磺酸与 12 种含氮化合物之间的氢键对大气的影响
IF 3
3区 化学
Q3 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
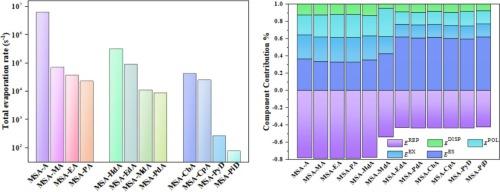
采用密度泛函理论、分子中的原子、基于局部分子轨道的能量分解分析和大气簇动态代码方法研究了甲磺酸(MSA)与氨(A)、烷基胺(aNHx)和环氨基化合物(cNHx)等 NHx 化合物之间的氢键相互作用。研究结果表明,这些二聚体通过 SOH⋯N 相互作用形成氢键。与 MSA-aNHx/A 二聚体相比,MSA-cNHx 二聚体的结合能更高。利用 AIM 进行的拓扑分析证实了这些二聚体中存在ρ(r) 和 ∇2ρ(r)氢键。IRI 的结果表明,这些二聚体中存在不同强度类型的氢键相互作用。LMO-EDA 强调静电相互作用是主要的吸引力,尤其是在 MSA-cNHx 二聚体中。ACDC 结果显示,与其他二聚体相比,MSA-CNHx 二聚体的蒸发率较低。这些发现表明,MSA 在 NPF 事件中起着关键作用,MSA-CNHx 团簇有可能成为大气中的成核。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Atmospheric implications of hydrogen bonding between methanesulfonic acid and 12 kinds of N-containing compounds
The hydrogen bonding interactions between methanesulfonic acid (MSA) and NHx compounds, such as ammonia (A), alkylamines (aNHx), and cyclic amino compounds (cNHx), were investigated using density functional theory, atoms in molecules, localized molecular orbitals-based energy decomposition analysis, and atmospheric clusters dynamic code methods. The results revealed that these dimers exhibit hydrogen bonds by SO![]() H⋯N interactions. MSA–cNHx dimers showed higher binding energies compared to MSA–aNHx/A dimers. Topological analysis using AIM confirmed the presence of hydrogen bonding in these dimers by ρ(r) and ∇2ρ(r). The results of IRI indicate that there are different strength types of hydrogen bonding interactions in these dimers. LMO–EDA highlighted electrostatic interactions as the main attractive force, particularly in MSA–cNHx dimers. ACDC results showed a low evaporation rate for MSA–cNHx dimers compared to others. These findings suggest that MSA plays a crucial role in NPF events, and MSA–cNHx clusters could potentially act as nucleation nuclei in the atmosphere.
H⋯N interactions. MSA–cNHx dimers showed higher binding energies compared to MSA–aNHx/A dimers. Topological analysis using AIM confirmed the presence of hydrogen bonding in these dimers by ρ(r) and ∇2ρ(r). The results of IRI indicate that there are different strength types of hydrogen bonding interactions in these dimers. LMO–EDA highlighted electrostatic interactions as the main attractive force, particularly in MSA–cNHx dimers. ACDC results showed a low evaporation rate for MSA–cNHx dimers compared to others. These findings suggest that MSA plays a crucial role in NPF events, and MSA–cNHx clusters could potentially act as nucleation nuclei in the atmosphere.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Computational and Theoretical Chemistry
CHEMISTRY, PHYSICAL-
CiteScore
4.20
自引率
10.70%
发文量
331
审稿时长
31 days
期刊介绍:
Computational and Theoretical Chemistry publishes high quality, original reports of significance in computational and theoretical chemistry including those that deal with problems of structure, properties, energetics, weak interactions, reaction mechanisms, catalysis, and reaction rates involving atoms, molecules, clusters, surfaces, and bulk matter.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


