苯并三嗪酮与α-烯烃进行脱氮烷基化的烯烃氢硼化/铃木偶联顺序法
IF 2.1
3区 化学
Q3 CHEMISTRY, INORGANIC & NUCLEAR
引用次数: 0
摘要
据报道,使用现成的 α-烯烃作为烷基来源,通过硼氢化/钯催化的烷基铃木偶联顺序,对 N-芳基苯并三嗪酮进行脱氮烷基化反应,可以得到正交 n-烷基苯甲酰胺,收率良好甚至极佳。对氢硼化/铃木偶联顺序的范围和局限性进行了探讨,结果表明苯并三嗪酮核心和烷基硼烷都有很大的立体效应。只有来自 α-olefins 的反马尔科夫尼科夫硼化反应的三烷基硼烷的伯烷基才能有效偶联,这使得该方案对正交正烷基苯甲酰胺具有高度选择性。本文章由计算机程序翻译,如有差异,请以英文原文为准。
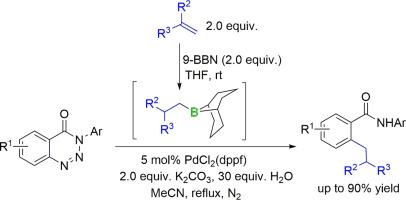
A sequential olefin hydroboration/Suzuki coupling for denitrogenative alkylation of benzotriazinones with α-olefins
A denitrogenative alkylation of N-aryl benzotriazinones using readily available α-olefins as alkyl sources via a hydroboration/palladium-catalyzed alkyl Suzuki coupling sequence is reported to afford ortho n-alkyl benzamides in good to excellent yields. Scope and limitations of the sequential hydroboration/Suzuki coupling protocol have been explored, showing large steric effects of both benzotriazinone core and alkylboranes. Only primary alkyl group of trialkylboranes from anti-Markovnikov hydroboration of α-olefins could be effectively coupled, making the protocol highly selective for ortho n-alkyl benzamides.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Organometallic Chemistry
化学-无机化学与核化学
CiteScore
4.40
自引率
8.70%
发文量
221
审稿时长
36 days
期刊介绍:
The Journal of Organometallic Chemistry targets original papers dealing with theoretical aspects, structural chemistry, synthesis, physical and chemical properties (including reaction mechanisms), and practical applications of organometallic compounds.
Organometallic compounds are defined as compounds that contain metal - carbon bonds. The term metal includes all alkali and alkaline earth metals, all transition metals and the lanthanides and actinides in the Periodic Table. Metalloids including the elements in Group 13 and the heavier members of the Groups 14 - 16 are also included. The term chemistry includes syntheses, characterizations and reaction chemistry of all such compounds. Research reports based on use of organometallic complexes in bioorganometallic chemistry, medicine, material sciences, homogeneous catalysis and energy conversion are also welcome.
The scope of the journal has been enlarged to encompass important research on organometallic complexes in bioorganometallic chemistry and material sciences, and of heavier main group elements in organometallic chemistry. The journal also publishes review articles, short communications and notes.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


