具有笨重取代基的有机锡羧酸盐。合成、结构、细胞毒性和抗氧化活性
IF 2.7
3区 化学
Q2 CHEMISTRY, INORGANIC & NUCLEAR
引用次数: 0
摘要
合成了一系列有机锡羧酸盐,并通过核磁共振(1H、13C、119Sn)和红外光谱、ESI 质谱和元素分析对其进行了表征。利用 X 射线衍射分析直接解析了两种化合物的结构,并发现了它们不同寻常的七配位 Sn 配位多面体。利用 DPPH、NBT 和 CUPRAC 测试、脂质过氧化和脂氧合酶抑制能力评估了合成化合物的抗氧化活性。结果表明,配体芳香环中羟基的存在极大地提高了复合物的抗氧化能力,但对抗增殖特性的影响并不明显,抗增殖特性是通过标准的 MTT 试验测定的。此外,研究表明二丁基锡和二叔丁基锡的衍生物具有最高的细胞毒性。研究人员将三种复合物作为先导化合物,并进行了更多的细胞凋亡诱导研究。复合物 4 和 7 显示出明显的 caspase 激活作用,从而确定了它们的作用模式。研究结果表明,上述复合物是很有前途的抗增殖剂。本文章由计算机程序翻译,如有差异,请以英文原文为准。
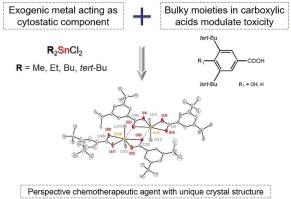
Organotin carboxylates with bulky substituents. Synthesis, structure, cytotoxicity and antioxidant activity
A series of organotin carboxylates were synthesized and characterized by NMR (1H, 13C, 119Sn) and IR spectroscopy, ESI mass-spectrometry and elemental analysis. The structure of two compounds was resolved directly using X-ray diffraction analysis and unusual heptacoordinated Sn coordination polyhedron was discovered for them. Antioxidant activity of the synthesized compounds was estimated using DPPH, NBT and CUPRAC-tests, lipid peroxidation and lipoxygenase inhibition capacity as well. It was shown that the presence of the hydroxyl group in the aromatic ring of the ligand drastically increases antioxidant potency of the complexes, while not noticeably affecting the antiproliferative properties, which were measured with the standard MTT-test. Moreover, it was shown that derivatives of dibutyl- and di-tert-butyltin exhibit the highest cytotoxicity. Three complexes were put forward as lead compounds and additional apoptosis induction studies were carried out. Noticeable caspase activation was shown for the complexes 4 and 7 thus marking their mode of action. The results obtained show that the complexes herein described are promising antiproliferative agents.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Inorganica Chimica Acta
化学-无机化学与核化学
CiteScore
6.00
自引率
3.60%
发文量
440
审稿时长
35 days
期刊介绍:
Inorganica Chimica Acta is an established international forum for all aspects of advanced Inorganic Chemistry. Original papers of high scientific level and interest are published in the form of Articles and Reviews.
Topics covered include:
• chemistry of the main group elements and the d- and f-block metals, including the synthesis, characterization and reactivity of coordination, organometallic, biomimetic, supramolecular coordination compounds, including associated computational studies;
• synthesis, physico-chemical properties, applications of molecule-based nano-scaled clusters and nanomaterials designed using the principles of coordination chemistry, as well as coordination polymers (CPs), metal-organic frameworks (MOFs), metal-organic polyhedra (MPOs);
• reaction mechanisms and physico-chemical investigations computational studies of metalloenzymes and their models;
• applications of inorganic compounds, metallodrugs and molecule-based materials.
Papers composed primarily of structural reports will typically not be considered for publication.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


