iNKT 细胞过度激活会加重支气管肺发育不良小鼠的肺损伤。
IF 10.7
1区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
支气管肺发育不良(BPD)是早产儿的一种严重肺部疾病,II型上皮细胞(AEC II)的异常增殖和分化能力是 BPD 病理基础的关键。AEC II 在 BPD 中的异常机制尚不清楚。本研究利用公开数据集、临床样本、高氧诱导的 BPD 小鼠模型和 AEC II-iNKT 细胞经孔共培养系统,研究了不变自然杀伤 T 细胞(iNKT)在 BPD 肺部疾病中的作用和机制。首先,我们发现在公共数据库中,BPD 患者的 NKT 细胞发育因子 IL-15 随时间推移而增加,临床采集的 BPD 患者外周血 NKT 细胞也有所增加。随后,与常氧组相比,高氧组的 iNKT 细胞比例增加,在 P7 时最高,并伴随着肺发育异常的活化增加。给予抗CD1d中和抗体抑制iNKT细胞可缓解高氧组小鼠肺发育异常,而给予α-GalCer可加重高氧组小鼠的肺损伤,收养性转移iNKT细胞可加重高氧组小鼠肺发育异常。此外,为了进一步验证 iNKT 细胞对 AEC II 的作用,建立了 AEC II-iNKT 细胞共培养系统。iNKT细胞的存在可加剧高氧条件下SP-C和T1α的异常表达。同时,RNA-seq分析表明,在高氧条件下,与iNKT细胞共培养的AEC II中铁突变相关基因高表达。我们进一步验证了高氧环境下 iNKT 细胞的存在对 AEC II 铁突变水平的影响,结果表明 iNKT 细胞在高氧环境下促进了 AEC II 的铁突变,并伴随着 SP-C 和 T1α 表达的降低。我们的研究发现,iNKT细胞在肺部的招募可能是导致BPD肺泡化障碍的重要原因。本文章由计算机程序翻译,如有差异,请以英文原文为准。
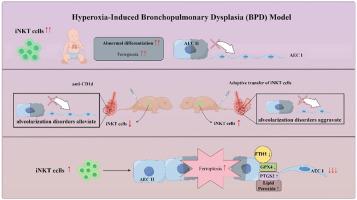
Over-activation of iNKT cells aggravate lung injury in bronchopulmonary dysplasia mice
Bronchopulmonary dysplasia (BPD) is a severe lung disease in preterm infants, the abnormal proliferate and differentiate ability of type II epithelial cells (AEC II) is the key to the pathological basis of BPD. Mechanisms regarding abnormal AEC II in BPD remain unclear. The present work investigated the role and mechanisms of invariant natural killer T (iNKT) cells in lung disorder in BPD using public datasets, clinical samples, a hyperoxia-induced BPD mouse model and AEC II-iNKT cells transwell co-culture system. Firstly, we found that the NKT cells development factor IL-15 increased over time in patients with BPD in public databases, and clinically collected peripheral blood NKT cells in patients with BPD were increased. Subsequently, the percentage of iNKT cells increased in hyperoxia group compared with normoxia group, with the highest at P7, accompanied by increased activation with abnormal lung development. The administration of anti-CD1d neutralizing antibody to inhibit iNKT cells could alleviate the abnormal lung development of hyperoxia group mice, while α-GalCer administration could aggravate lung injury in hyperoxia group mice, and adoptive transfer of iNKT cells could aggravate the abnormal lung development in hyperoxia group mice. In addition, to further verify the role of iNKT cells on AEC II, AEC II-iNKT cells co-culture system was established. The presence of iNKT cells could aggravate the abnormal expression of SP-C and T1α under hyperoxia. Meanwhile, RNA-seq analysis showed that ferroptosis-related genes were highly expressed in AEC II co-cultured with iNKT cells under hyperoxia. We further validated the effect of the presence of iNKT cells under hyperoxia environment on AEC II ferroptosis levels, suggested that iNKT cells promote AEC II ferroptosis under hyperoxia, accompanied by decreased expression of SP-C and T1α. Our study found that the recruitment of iNKT cells in the lung may be an important cause of alveolarization disorder in BPD.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Redox Biology
BIOCHEMISTRY & MOLECULAR BIOLOGY-
CiteScore
19.90
自引率
3.50%
发文量
318
审稿时长
25 days
期刊介绍:
Redox Biology is the official journal of the Society for Redox Biology and Medicine and the Society for Free Radical Research-Europe. It is also affiliated with the International Society for Free Radical Research (SFRRI). This journal serves as a platform for publishing pioneering research, innovative methods, and comprehensive review articles in the field of redox biology, encompassing both health and disease.
Redox Biology welcomes various forms of contributions, including research articles (short or full communications), methods, mini-reviews, and commentaries. Through its diverse range of published content, Redox Biology aims to foster advancements and insights in the understanding of redox biology and its implications.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


