洞察 E2F5 条件性基因敲除小鼠模型中的乳腺发育和肿瘤进展。
IF 6.9
1区 医学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
乳腺癌的发生与乳腺发育过程的调节改变有关。因此,更好地了解乳腺的正常发育过程可以揭示正常细胞如何被重新编程成为恶性细胞的可能机制。E2F 1-4 属于 E2F 转录因子家族,在乳腺发育过程中发挥着不同的作用,但人们对 E2F5 的作用知之甚少。scRNAseq和预测特征工具的组合证明了E2F5在乳腺中的存在,并显示了在乳腺发育的不同阶段预测活性的变化。为了验证 E2F5 调控乳腺功能的假设,我们建立了一个乳腺特异性 E2F5 基因敲除小鼠模型,结果发现小鼠的乳腺发育变化不大。然而,经过长期潜伏后,E2F5条件性基因敲除小鼠出现了高度转移性乳腺肿瘤。全基因组测序揭示了肿瘤间的显著异质性。RNAseq和蛋白质分析发现,Cyclin D1的水平发生了改变,与MMTV-Neu肿瘤相似,这表明E2F5条件性基因敲除小鼠的乳腺和肿瘤可能依赖于Cyclin D1。肿瘤移植后会发现淋巴结转移,在免疫功能正常的受体中进行连续移植后,淋巴结转移会更多。基于这些发现,我们认为 E2F5 的缺失会导致 Cyclin D1 的调控发生改变,从而在长期潜伏后促进转移性乳腺肿瘤的发展。更重要的是,这项研究表明,乳腺中条件性 E2F5 的缺失会导致肿瘤的形成,从而揭示了它作为转录因子调控基因网络的作用,而这种调控通常会产生肿瘤抑制功能。本文章由计算机程序翻译,如有差异,请以英文原文为准。
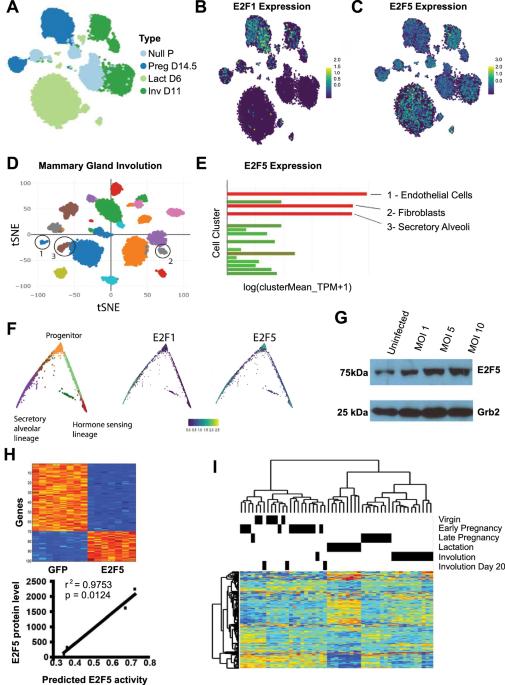
Insight into mammary gland development and tumor progression in an E2F5 conditional knockout mouse model
Development of breast cancer is linked to altered regulation of mammary gland developmental processes. A better understanding of normal mammary gland development can thus reveal possible mechanisms of how normal cells are re-programmed to become malignant. E2Fs 1-4 are part of the E2F transcription factor family with varied roles in mammary development, but little is known about the role of E2F5. A combination of scRNAseq and predictive signature tools demonstrated the presence of E2F5 in the mammary gland and showed changes in predicted activity during the various phases of mammary gland development. Testing the hypothesis that E2F5 regulates mammary function, we generated a mammary-specific E2F5 knockout mouse model, resulting in modest mammary gland development changes. However, after a prolonged latency the E2F5 conditional knockout mice developed highly metastatic mammary tumors. Whole genome sequencing revealed significant intertumor heterogeneity. RNAseq and protein analysis identified altered levels of Cyclin D1, with similarities to MMTV-Neu tumors, suggesting that E2F5 conditional knockout mammary glands and tumors may be dependent on Cyclin D1. Transplantation of the tumors revealed metastases to lymph nodes that were enriched through serial transplantation in immune competent recipients. Based on these findings, we propose that loss of E2F5 leads to altered regulation of Cyclin D1, which facilitates the development of metastatic mammary tumors after long latency. More importantly, this study demonstrates that conditional loss of E2F5 in the mammary gland leads to tumor formation, revealing its role as a transcription factor regulating a network of genes that normally result in a tumor suppressor function.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Oncogene
医学-生化与分子生物学
CiteScore
15.30
自引率
1.20%
发文量
404
审稿时长
1 months
期刊介绍:
Oncogene is dedicated to advancing our understanding of cancer processes through the publication of exceptional research. The journal seeks to disseminate work that challenges conventional theories and contributes to establishing new paradigms in the etio-pathogenesis, diagnosis, treatment, or prevention of cancers. Emphasis is placed on research shedding light on processes driving metastatic spread and providing crucial insights into cancer biology beyond existing knowledge.
Areas covered include the cellular and molecular biology of cancer, resistance to cancer therapies, and the development of improved approaches to enhance survival. Oncogene spans the spectrum of cancer biology, from fundamental and theoretical work to translational, applied, and clinical research, including early and late Phase clinical trials, particularly those with biologic and translational endpoints.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


