蛋白激酶 N1 缺乏会导致脑能量代谢上调,并在体内和体外中风模型中具有高度保护作用。
IF 10.8
1区 医学
Q1 ENDOCRINOLOGY & METABOLISM
引用次数: 0
摘要
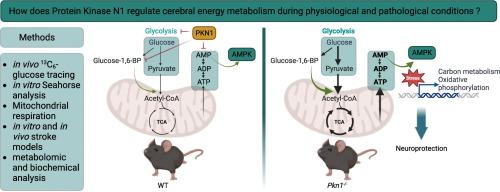
背景和目的:我们最近发现蛋白激酶 N1(PKN1)是大脑发育的主要调节因子。然而,它在成人大脑中的功能尚未明确确定。在这项研究中,我们评估了野生型(WT)和全基因Pkn1敲除(Pkn1-/-)动物在生理和病理生理条件下的脑能量表型:方法:通过体内13C6-葡萄糖追踪和体外实时海马分析细胞外酸化率以及脑切片冲片线粒体耗氧率(OCR)来分析脑能量代谢。对分离的 WT 和 Pkn1-/- 脑线粒体进行了测试,以确定不同底物在 OCR 方面的差异。在缺血性中风的体外模型中,通过氧-葡萄糖剥夺和再灌注诱导的控制和能量应激条件下,通过质谱分析测定脑片中的代谢物水平。酶测定、Western 印迹和大量 RNA 测序评估了酶活性的差异。使用大脑中动脉闭塞中风模型分析 WT 小鼠和 Pkn1-/- 小鼠的病变体积和功能恢复情况:结果:Pkn1 缺乏导致体内和体外大脑能量代谢显著上调。这是由于两种不同的机制造成的,其中包括糖酵解通量的增强和丙酮酸诱导的线粒体 OCR 的增加。从机理上讲,我们发现 Pkn1-/- 脑组织的糖酵解限速酶磷酸果激酶的活性增加。此外,Pkn1 缺乏时,1,6-二磷酸葡萄糖(一种可增加线粒体丙酮酸摄取的代谢产物)水平升高。因此,在能量应激期间,Pkn1-/-脑片有更多的 ATP 和更多的 ATP 降解代谢物积累。这转化为体外中风期间单磷酸腺苷(AMP)激活的蛋白激酶(AMPK)的磷酸化和活性增加。因此,Pkn1-/-脑片显示出缺血后能量代谢途径的转录上调,Pkn1的缺乏在体外和体内中风模型中具有很强的保护作用。虽然抑制线粒体丙酮酸摄取对保护性表型影响不大,但抑制Pkn1-/-脑片中的AMPK会增加体外缺血后的细胞死亡:这是首次全面证明 PKN1 在脑能量代谢中的重要和独特作用的研究,它同时调节糖酵解和线粒体丙酮酸诱导的呼吸。我们还进一步发现了在体外和体内中风模型中缺乏 Pkn1 的高度保护性表型,从而验证了抑制 PKN1 是开发新型中风疗法的一个很有前景的新治疗靶点。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Protein kinase N1 deficiency results in upregulation of cerebral energy metabolism and is highly protective in in vivo and in vitro stroke models
Background and aim
We recently identified protein kinase N1 (PKN1) as a master regulator of brain development. However, its function in the adult brain has not been clearly established. In this study, we assessed the cerebral energetic phenotype of wildtype (WT) and global Pkn1 knockout (Pkn1−/−) animals under physiological and pathophysiological conditions.
Methods
Cerebral energy metabolism was analyzed by 13C6-glucose tracing in vivo and real time seahorse analysis of extracellular acidification rates as well as mitochondrial oxygen consumption rates (OCR) of brain slice punches in vitro. Isolated WT and Pkn1−/− brain mitochondria were tested for differences in OCR with different substrates. Metabolite levels were determined by mass spectrometric analysis in brain slices under control and energetic stress conditions, induced by oxygen-glucose deprivation and reperfusion, an in vitro model of ischemic stroke. Differences in enzyme activities were assessed by enzymatic assays, western blotting and bulk RNA sequencing. A middle cerebral artery occlusion stroke model was used to analyze lesion volumes and functional recovery in WT and Pkn1−/− mice.
Results
Pkn1 deficiency resulted in a remarkable upregulation of cerebral energy metabolism, in vivo and in vitro. This was due to two separate mechanisms involving an enhanced glycolytic flux and higher pyruvate-induced mitochondrial OCR. Mechanistically we show that Pkn1−/− brain tissue exhibits an increased activity of the glycolysis rate-limiting enzyme phosphofructokinase. Additionally, glucose-1,6-bisphosphate levels, a metabolite that increases mitochondrial pyruvate uptake, were elevated upon Pkn1 deficiency. Consequently, Pkn1−/− brain slices had more ATP and a greater accumulation of ATP degradation metabolites during energetic stress. This translated into increased phosphorylation and activity of adenosine monophosphate (AMP)-activated protein kinase (AMPK) during in vitro stroke. Accordingly, Pkn1−/− brain slices showed a post-ischemic transcriptional upregulation of energy metabolism pathways and Pkn1 deficiency was strongly protective in in vitro and in vivo stroke models. While inhibition of mitochondrial pyruvate uptake only moderately affected the protective phenotype, inhibition of AMPK in Pkn1−/− slices increased post-ischemic cell death in vitro.
Conclusion
This is the first study to comprehensively demonstrate an essential and unique role of PKN1 in cerebral energy metabolism, regulating glycolysis and mitochondrial pyruvate-induced respiration. We further uncovered a highly protective phenotype of Pkn1 deficiency in both, in vitro and in vivo stroke models, validating inhibition of PKN1 as a promising new therapeutic target for the development of novel stroke therapies.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Metabolism: clinical and experimental
医学-内分泌学与代谢
CiteScore
18.90
自引率
3.10%
发文量
310
审稿时长
16 days
期刊介绍:
Metabolism upholds research excellence by disseminating high-quality original research, reviews, editorials, and commentaries covering all facets of human metabolism.
Consideration for publication in Metabolism extends to studies in humans, animal, and cellular models, with a particular emphasis on work demonstrating strong translational potential.
The journal addresses a range of topics, including:
- Energy Expenditure and Obesity
- Metabolic Syndrome, Prediabetes, and Diabetes
- Nutrition, Exercise, and the Environment
- Genetics and Genomics, Proteomics, and Metabolomics
- Carbohydrate, Lipid, and Protein Metabolism
- Endocrinology and Hypertension
- Mineral and Bone Metabolism
- Cardiovascular Diseases and Malignancies
- Inflammation in metabolism and immunometabolism
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


