论球蛋白中 AXXXA 序列基序的丰度和重要性及其在 CβCβ 相互作用中的参与。
IF 2.7
3区 生物学
Q3 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
AXXXA 和 GXXXG 主题经常在螺旋中出现,尤其是在膜蛋白中。已知 GXXXG 主题通过 CHO 键稳定膜蛋白中的螺旋-螺旋结合。此外,AXXXA 序列主题还能稳定蛋白质的折叠状态。我们发现,在一组非冗余的 6000 个强制性同源二聚体(OD)复合物中,AXXXA 和 GXXXG 主题分别出现了 27,000 和 18,000 次。有趣的是,这种情况在瞬时同源二聚体(TD)和异源二聚体(HetD)中并不明显。每个同源二聚体平均含有四个 AXXXA 矩阵,HetD 和 TD 分别为 2 个和 3.5 个。从结合表面可以看出,27%的 OD 在界面上至少含有一个 AXXXA 基序,而 HetD 和 TD 的这一比例分别为 17% 和 15%。AXXXA 主要通过侧链 CβCβ 相互作用来稳定 OD 的四元结构。这种相互作用在能量上是有利的,是 OD 四元结构稳定性的主要驱动力。观察到的 Cβ-Cβ 相互作用比已知的用于稳定螺旋-螺旋的 CHO 相互作用高出 6 倍。在含有 AXXXA 的界面区域还观察到 CβO 和 OO 的两种新的相互作用。如果任何一个末端的 Ala 残基被 Gly 取代,则该图案的出现率会急剧下降。我们的研究结果表明了 AXXXA 在通过特异性疏水相互作用为四元结构提供稳定性方面的重要性,以及图案末端 Ala 残基的特异性。所获得的知识可用于设计稳定性更好的合成蛋白质和设计基于肽的疗法。本文章由计算机程序翻译,如有差异,请以英文原文为准。
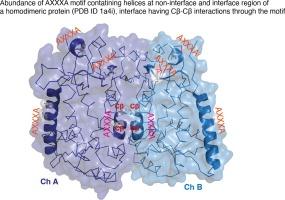
On the abundance and importance of AXXXA sequence motifs in globular proteins and their involvement in CβCβ interaction
The AXXXA and GXXXG motifs are frequently observed in helices, especially in membrane proteins. The motif GXXXG is known to stabilize helix-helix association in membrane proteins via CαH![]() O bonding. AXXXA sequence motif additionally stabilizes the folded state of proteins. We found 27,000 and 18,000 occurrences of AXXXA and GXXXG motifs in a non-redundant set of 6000 obligate homodimeric (OD) complexes. Interestingly, this is less pronounced in transient homodimers (TD) and heterodimers (HetD). On average each obligate homodimer contains four AXXXA motifs, it is 2 and 3.5 for HetD and TD, respectively. Focusing on the binding surface it is seen that 27 % of the ODs contain at least one AXXXA motif at the interface, whereas it is 17 % and 15 % for HetD and TD respectively. AXXXA predominantly stabilizes the OD quaternary structure via the side chain Cβ
O bonding. AXXXA sequence motif additionally stabilizes the folded state of proteins. We found 27,000 and 18,000 occurrences of AXXXA and GXXXG motifs in a non-redundant set of 6000 obligate homodimeric (OD) complexes. Interestingly, this is less pronounced in transient homodimers (TD) and heterodimers (HetD). On average each obligate homodimer contains four AXXXA motifs, it is 2 and 3.5 for HetD and TD, respectively. Focusing on the binding surface it is seen that 27 % of the ODs contain at least one AXXXA motif at the interface, whereas it is 17 % and 15 % for HetD and TD respectively. AXXXA predominantly stabilizes the OD quaternary structure via the side chain Cβ![]() Cβ interactions. This interaction is energetically favorable and is found to be a major driving force for OD quaternary structure stability. Cβ-Cβ interactions are observed ∼6 times higher than the known CαH
Cβ interactions. This interaction is energetically favorable and is found to be a major driving force for OD quaternary structure stability. Cβ-Cβ interactions are observed ∼6 times higher than the known CαH![]() O interaction for helix-helix stabilization. Two additional new interactions of Cβ
O interaction for helix-helix stabilization. Two additional new interactions of Cβ![]() O and O
O and O![]() O are observed at the AXXXA containing interface regions. The occurrence of the motif gets drastically reduced if any of the terminal Ala residues are replaced by Gly. Our findings show the importance of AXXXA in providing stability to the quaternary structure through specific hydrophobic interactions and the specificity of the Ala residue at motif termini. The knowledge gained can be used for designing synthetic proteins of improved stability and for designing peptide-based therapeutics.
O are observed at the AXXXA containing interface regions. The occurrence of the motif gets drastically reduced if any of the terminal Ala residues are replaced by Gly. Our findings show the importance of AXXXA in providing stability to the quaternary structure through specific hydrophobic interactions and the specificity of the Ala residue at motif termini. The knowledge gained can be used for designing synthetic proteins of improved stability and for designing peptide-based therapeutics.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of structural biology
生物-生化与分子生物学
CiteScore
6.30
自引率
3.30%
发文量
88
审稿时长
65 days
期刊介绍:
Journal of Structural Biology (JSB) has an open access mirror journal, the Journal of Structural Biology: X (JSBX), sharing the same aims and scope, editorial team, submission system and rigorous peer review. Since both journals share the same editorial system, you may submit your manuscript via either journal homepage. You will be prompted during submission (and revision) to choose in which to publish your article. The editors and reviewers are not aware of the choice you made until the article has been published online. JSB and JSBX publish papers dealing with the structural analysis of living material at every level of organization by all methods that lead to an understanding of biological function in terms of molecular and supermolecular structure.
Techniques covered include:
• Light microscopy including confocal microscopy
• All types of electron microscopy
• X-ray diffraction
• Nuclear magnetic resonance
• Scanning force microscopy, scanning probe microscopy, and tunneling microscopy
• Digital image processing
• Computational insights into structure
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


