经颅直流电刺激作为一种潜在的脱髓鞘疗法:铜绿素脱髓鞘的视觉诱发电位恢复。
IF 4.6
2区 医学
Q1 NEUROSCIENCES
引用次数: 0
摘要
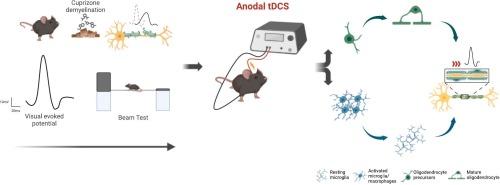
目的:据报道,经颅直流电刺激(tDCS)对神经元的可塑性具有有益的影响,因此已被提议作为促进多种神经系统疾病(包括多发性硬化症等脱髓鞘疾病)功能恢复的一种治疗方法。有关 tDCS 对多发性硬化症主要病理机制(如脱髓鞘和炎症)的影响的信息较少。为了更多地了解后者的影响,我们在长期食用铜松(CPZ)的小鼠身上应用了多节阳极tDCS,众所周知,铜松会诱发慢性脱髓鞘:方法:采用视觉诱发电位(VEP)和运动表现(光束测试)对28只接受CPZ饮食的小鼠和12只对照组(H)小鼠的视觉和运动通路进行纵向监测。随机分组后,对清醒、自由活动的存活小鼠进行为期 5 天的阳极 tDCS 治疗:12 只 CPZ-anodal、10 只 CPZ-sham、5 只 H-anodal、5 只 h-sham。实验结束后,对视神经和胼胝体的髓鞘、轴突和小胶质细胞/巨噬细胞进行组织学分析:主要发现:与基线相比,CPZ 饮食会导致 4 周开始的 VEPs 明显延迟,与对照组相比,8 周开始的 VEPs 明显延迟。经过 5 天的 tDCS 后,活性组的 VEPs 潜伏期比假组有明显恢复。在光束交叉时间测试中也观察到了类似的结果。视神经组织学显示,CPZ-Anodal 组与 CPZ-Sham 组相比,髓鞘含量更高,小胶质细胞/巨噬细胞数量更少:对自由活动的小鼠进行多次阳极经颅直流电刺激(tDCS)可恢复视觉神经传导,并对铜绿素脱髓鞘模型中的髓鞘含量和炎性细胞产生显著的有益影响。总之,这些令人鼓舞的发现促使人们进一步探索将 tDCS 作为髓鞘再形成的潜在治疗方法。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Transcranial direct current stimulation as a potential remyelinating therapy: Visual evoked potentials recovery in cuprizone demyelination
Aims
Non-invasive neuromodulation by transcranial direct current stimulation (tDCS), owing to its reported beneficial effects on neuronal plasticity, has been proposed as a treatment to promote functional recovery in several neurological conditions, including demyelinating diseases like multiple sclerosis. Less information is available on the effects of tDCS in major pathological mechanisms of multiple sclerosis, such as demyelination and inflammation. To learn more about the latter effects, we applied multi-session anodal tDCS in mice exposed to long-term cuprizone (CPZ) diet, known to induce chronic demyelination.
Methods
Visual evoked potentials (VEP) and motor performance (beam test) were employed for longitudinal monitoring of visual and motor pathways in 28 mice undergoing CPZ diet, compared with 12 control (H) mice. After randomization, anodal tDCS was applied for 5 days in awake, freely-moving surviving animals: 12 CPZ-anodal, 10 CPZ-sham, 5H-anodal, 5 h-sham. At the end of the experiment, histological analysis was performed on the optic nerves and corpus callosum for myelin, axons and microglia/macrophages.
Key findings
CPZ diet was associated with significantly delayed VEPs starting at 4 weeks compared with their baseline, significant compared with controls at 8 weeks. After 5-day tDCS, VEPs latency significantly recovered in the active group compared with the sham group. Similar findings were observed in the time to cross on the beam test Optic nerve histology revealed higher myelin content and lower microglia/macrophage counts in the CPZ-Anodal group compared with CPZ-Sham.
Significance
Multiple sessions of anodal transcranial direct current stimulation (tDCS) in freely moving mice induced recovery of visual nervous conduction and significant beneficial effects in myelin content and inflammatory cells in the cuprizone model of demyelination. Altogether, these promising findings prompt further exploration of tDCS as a potential therapeutic approach for remyelination.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Experimental Neurology
医学-神经科学
CiteScore
10.10
自引率
3.80%
发文量
258
审稿时长
42 days
期刊介绍:
Experimental Neurology, a Journal of Neuroscience Research, publishes original research in neuroscience with a particular emphasis on novel findings in neural development, regeneration, plasticity and transplantation. The journal has focused on research concerning basic mechanisms underlying neurological disorders.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


