神经退行性疾病中谷氨酸和 tau 介导的毒性早期阶段的共同和不同途径。
IF 4.6
2区 医学
Q1 NEUROSCIENCES
引用次数: 0
摘要
研究表明,兴奋性毒性和 tau 介导的毒性是导致阿尔茨海默病(AD)神经元死亡的主要因素。兴奋性氨基酸转运体 2(EAAT2 或 GLT-1)是大脑中调节突触内和突触外谷氨酸水平的主要谷氨酸转运体。同样,神经纤维缠结(由过度磷酸化的 tau 蛋白组成)的形成也与认知能力下降和神经元萎缩有关。然而,对上述毒性起关键作用的共同基因和途径尚未得到很好的阐明。为了研究谷氨酸失衡和 tau 积累对受影响的海马神经元翻译谱的影响,我们使用小鼠兴奋毒性模型和 tau 介导的毒性模型(分别为 GLT-1-/ 和 P301S),并结合 BAC-TRAP 技术。我们的数据显示,CA3 锥体神经元中 GLT-1 的缺乏会导致与突触可塑性和神经元存活相关的通路失调的翻译特征,而 P301S 突变会诱导内细胞通路的变化和线粒体功能障碍。最后,常见的失调途径包括离子平衡受损和代谢途径。这些常见途径可能为改善谷氨酸和tau介导的AD毒性提供了潜在的治疗靶点。本文章由计算机程序翻译,如有差异,请以英文原文为准。
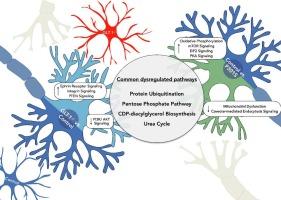
Common and divergent pathways in early stages of glutamate and tau-mediated toxicities in neurodegeneration
It has been shown that excitotoxicity and tau-mediated toxicities are major contributing factors to neuronal death in Alzheimer's disease (AD). The excitatory amino acid transporter 2 (EAAT2 or GLT-1), the major glutamate transporter in the brain that regulates glutamate levels synaptically and extrasynaptically, has been shown to be deficient in AD brains, leading to excitotoxicity and subsequent cell death. Similarly, buildup of neurofibrillary tangles, which consist of hyperphosphorylated tau protein, correlates with cognitive decline and neuronal atrophy in AD. However, common genes and pathways that are critical in the aforementioned toxicities have not been well elucidated. To investigate the impact of glutamate dyshomeostasis and tau accumulation on translational profiles of affected hippocampal neurons, we used mouse models of excitotoxicity and tau–mediated toxicities (GLT-1−/− and P301S, respectively) in conjunction with BAC-TRAP technology. Our data show that GLT-1 deficiency in CA3 pyramidal neurons leads to translational signatures characterized by dysregulation of pathways associated with synaptic plasticity and neuronal survival, while the P301S mutation induces changes in endocytic pathways and mitochondrial dysfunction. Finally, the commonly dysregulated pathways include impaired ion homeostasis and metabolic pathways. These common pathways may shed light on potential therapeutic targets for ameliorating glutamate and tau-mediated toxicities in AD.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Experimental Neurology
医学-神经科学
CiteScore
10.10
自引率
3.80%
发文量
258
审稿时长
42 days
期刊介绍:
Experimental Neurology, a Journal of Neuroscience Research, publishes original research in neuroscience with a particular emphasis on novel findings in neural development, regeneration, plasticity and transplantation. The journal has focused on research concerning basic mechanisms underlying neurological disorders.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


