以免疫疗法为基础的联合疗法相关评分是肾细胞癌中免疫疗法和血管内皮生长因子抑制剂联合疗法疗效和反应的可靠预测指标。
IF 7
2区 医学
Q1 BIOLOGY
引用次数: 0
摘要
背景:在过去的十年中,基于免疫疗法的肾细胞癌(RCC)综合疗法迅速发展,但迄今为止,其成功率仍然有限。这种局限性主要源于缺乏识别可能从此类疗法中获益的患者所必需的生物标志物:在这项研究中,基于加权相关网络分析(WGCNA)在 IMmotion151 数据集中提取的关键模块中确定的基因,采用单样本基因组富集分析(ssGSEA)建立了基于免疫疗法的联合相关评分(IBCS):结果:高IBCS患者对基于免疫疗法的联合疗法的反应更好,无进展生存期(PFS)更长。进一步的转录组分析表明,IBCS与TIDE评分呈负相关,确定了一个RCC患者亚群,其特点是T-效应基因表达丰富,细胞周期/血管生成基因表达适中。我们对枢纽基因的分析揭示了一种新型分子,它有可能成为 RCC 的靶抗原。通过在包含180个样本的组织芯片(TMA)上进行多重免疫荧光检测验证,证实了该中枢基因在免疫调节中的关键作用。此外,我们还建立了一个独立的风险评分模型,这对预后评估和患者分层具有重要意义。值得注意的是,我们利用这个风险评分模型设计了一个预测提名图,其预后准确性超过了 IMDC 评分(一个被广泛接受的预测血管内皮生长因子靶向治疗患者生存率的风险评分):本研究共同开发了基于免疫疗法的联合疗法相关评分,精确定位了肾癌患者预后和对基于免疫疗法的联合疗法反应性的有效生物标志物,并深入研究了其潜在的生物学机制,为进一步探索提供了有前景的靶点。本文章由计算机程序翻译,如有差异,请以英文原文为准。
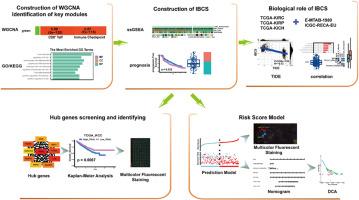
The immunotherapy-based combination associated score as a robust predictor for outcome and response to combination of immunotherapy and VEGF inhibitors in renal cell carcinoma
Background
Over the past decade, the realm of immunotherapy-based combination therapy has witnessed rapid growth for renal cell carcinoma (RCC), however, success has been constrained thus far. This limitation primarily stems from the absence of biomarkers essential for identifying patients likely to derive benefits from such treatments.
Methods
In this study, the immunotherapy-based combination associated score (IBCS) was established using single-sample gene set enrichment analysis (ssGSEA) based on the genes identified in the key modules extracted by weighted correlation network analysis (WGCNA) in the IMmotion151 dataset, a randomized, global phase III trial.
Results
High IBCS patients showed better responses to immunotherapy-based combinations and had longer progression-free survival (PFS). Further transcriptomic analysis revealed that IBCS was negatively correlated to TIDE score, identifying a subset of RCC patients characterized by enrichment of T-effector and moderate cell-cycle/angiogenesis gene expression. Our analysis of hub genes unveiled a novel molecule that could potentially serve as a target antigen in RCC. Validation through multiplex immunofluorescence assays on tissue microarrays (TMAs) containing 180 samples confirmed the pivotal role of this hub gene in immunoregulation. Furthermore, we developed an independent risk score model, which is significant for prognostic evaluation and patient stratification. Notably, we devised a forecasting nomogram using this risk score model, surpassing the IMDC score (a widely accepted risk score for predicting survival in patients undergoing VEGF-targeted therapy) in prognostic accuracy for patients treated with immunotherapy-based combinations.
Conclusion
This study has collectively developed an immunotherapy-based combination associated score, pinpointed effective biomarkers for prognostic and responsiveness of kidney cancer patients to immunotherapy-based combinations, and delved into their potential biological mechanisms, offering promising targets for further exploration.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Computers in biology and medicine
工程技术-工程:生物医学
CiteScore
11.70
自引率
10.40%
发文量
1086
审稿时长
74 days
期刊介绍:
Computers in Biology and Medicine is an international forum for sharing groundbreaking advancements in the use of computers in bioscience and medicine. This journal serves as a medium for communicating essential research, instruction, ideas, and information regarding the rapidly evolving field of computer applications in these domains. By encouraging the exchange of knowledge, we aim to facilitate progress and innovation in the utilization of computers in biology and medicine.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


