电压驱动的氨基甲酸乙酯通量导致氨基甲酸乙酯通道失活。
IF 2.5
3区 生物学
Q3 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
我们的研究表明,单靠电压就能使甲氧霉素通道失活,这在之前的单唑霉素和苏唑西林通道中也观察到过。触发失活所需的电压高于形成通道的电位,与通道激活一样,这一阈值随着肽浓度和膜流动性的增加而降低。由于类似的单霉素通道是通过通道断裂和转运而失活的,我们假设阿拉米霉素通道的失活也是通过同样的机制发生的。我们的数据通过两个实验证明了这一假设的正确性。首先,我们表明,在正电压下,当只向顺式侧提供肽时,通道失活与负电位时反式侧的新通道活性相关。这一结果表明,氨基甲酸乙酯肽的转位只发生在电压诱导的失活过程中。其次,我们测量了两侧含有等量氨甲蝶呤的膜的稳态(失活)与理想(无失活)电导与电压之比,并用这些值来量化氨甲蝶呤通量。将多种氨甲蝶呤浓度下的通量与稳态电导绘制成图,显示出单一的线性依赖关系,这表明转运的肽来自活性通道,而这些通道在长时间的电压作用下会断裂。鉴于氨甲蝶呤经常被用作离子通道模型,这些结果使人们对其在长时间高电压下的动力学反应有了更重要的了解。本文章由计算机程序翻译,如有差异,请以英文原文为准。
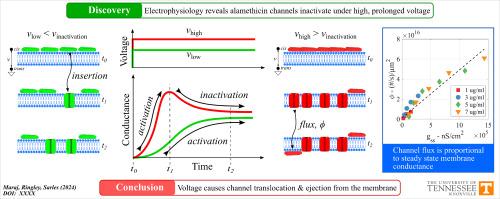
Alamethicin channel inactivation caused by voltage-driven flux of alamethicin
We show that voltage alone can inactivate alamethicin channels, which has been previously observed for monazomycin and suzukacillin channels. The voltage required to trigger inactivation is above the potential to form channels, and, like with channel activation, this threshold reduces with increasing peptide concentration and membrane fluidity. Since similar monazomycin channels inactivate via channel break up and translocation, we hypothesized that inactivation of alamethicin channels occurs via the same mechanism. Our data prove this hypothesis to be true through two experiments. First, we show that inactivation of channels at positive voltages when peptides are supplied to only the cis side correlates to new channel activity on the trans side at negative potentials. This result indicates translocation of alamethicin peptides occurs only during voltage-induced inactivation. Second, we measured the ratio of steady-state (with inactivation) to ideal (without inactivation) conductance versus voltage for membranes with equal amounts of alamethicin on both sides and used these values to quantify alamethicin flux. Plotting flux versus steady-state conductance across multiple alamethicin concentrations shows a single linear dependence, signifying that translocated peptides originate from active channels that break up under prolonged voltage. Given the frequent use of alamethicin as model ion channels, these results add important understanding of their kinetic responses when subjected to prolonged, high voltages.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Biochimica et biophysica acta. Biomembranes
生物-生化与分子生物学
CiteScore
8.20
自引率
5.90%
发文量
175
审稿时长
2.3 months
期刊介绍:
BBA Biomembranes has its main focus on membrane structure, function and biomolecular organization, membrane proteins, receptors, channels and anchors, fluidity and composition, model membranes and liposomes, membrane surface studies and ligand interactions, transport studies, and membrane dynamics.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


