组装双孢蘑菇中的 I 类亲水蛋白可产生不同的淀粉样纤维。
IF 2.3
4区 生物学
Q3 BIOCHEMISTRY & MOLECULAR BIOLOGY
Biochimica et biophysica acta. Proteins and proteomics
Pub Date : 2024-09-26
DOI:10.1016/j.bbapap.2024.141048
引用次数: 0
摘要
这项工作研究了从双孢蘑菇(ABH4)中提取、纯化、表征和组装疏水蛋白 I 类。从针头中获得的可溶性蛋白质浓度最高,对疏水素 I 的提取和纯化效率很高,获得了 12 kDa 的条带。经鉴定的疏水蛋白序列含有八个半胱氨酸残基;在结构预测方面,疏水蛋白的α螺旋结构多于β薄片结构。据观察,疏水素能分别减小和增大聚四氟乙烯和玻璃的接触角,发现胶束临界浓度为 221 μg mL-1。ThT 实验表明,在碱性 pH 值下,纤维的生成量减少,而酸性和中性 pH 值则有利于纤维的形成。同样,添加胶体聚四氟乙烯也会影响纤维的形成。圆二色性光谱证明,Ⅰ类疏水蛋白在涡旋后二级结构发生变化,α螺旋和β薄片含量增加。据观察,通过扫描电子显微镜和原子力显微镜分析,嗜水素在玻璃和云母中产生了不同的淀粉样结构。本文章由计算机程序翻译,如有差异,请以英文原文为准。
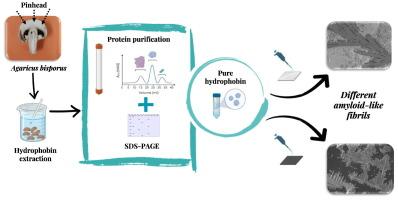
Assembly of Hydrophobin class I from Agaricus bisporus produced different amyloid-like fibrils
This work studied the extraction, purification, characterization, and assembly of hydrophobin class I from Agaricus bisporus (ABH4). The highest soluble protein concentration was obtained from the pinhead, the extraction and purification were efficient for hydrophobin class I, obtaining a band of 12 kDa. The identified sequence of hydrophobin presented the eight cysteine residues; for the prediction of the structure, hydrophobin presented more alpha helix structures than beta sheets. It was observed that the hydrophobin managed to decrease and increase the contact angle in Teflon and glass, respectively, finding a micellar critical concentration of 221 μg mL−1. ThT experiments demonstrated that the production of fibrils decreased at basic pH, while acidic and neutral pH favoured the formation of fibrils. Likewise, the addition of colloidal Teflon affects the formation of fibrils. Circular dichroism spectra proved that hydrophobin class I undergo changes in its secondary structure, increasing its alpha helix and beta sheet content after vortexing. It was observed that the analysis by scanning electron microscopy and atomic force microscopy of the hydrophobin produced different amyloid-like structures in glass and mica.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
8.00
自引率
0.00%
发文量
55
审稿时长
33 days
期刊介绍:
BBA Proteins and Proteomics covers protein structure conformation and dynamics; protein folding; protein-ligand interactions; enzyme mechanisms, models and kinetics; protein physical properties and spectroscopy; and proteomics and bioinformatics analyses of protein structure, protein function, or protein regulation.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


