Vilde E Skingen, Unn Beate Salberg, Tord Hompland, Christina S Fjeldbo, Hanna Helgeland, Kari-Anne M Frikstad, Harald B Ragnum, Ljiljana Vlatkovic, Knut Håkon Hole, Therese Seierstad, Heidi Lyng
下载PDF
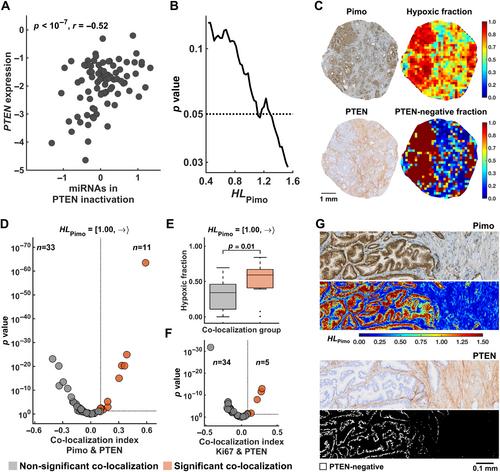
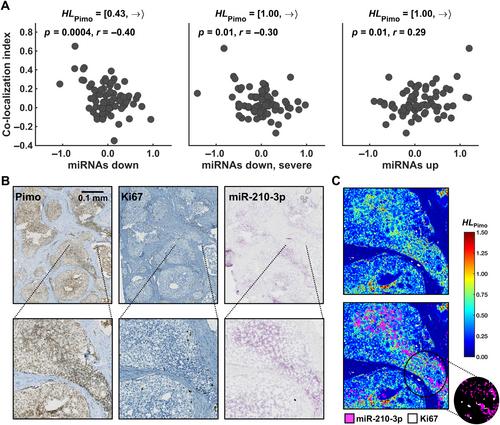
{"title":"在确定的肿瘤缺氧水平下对微RNA调控的空间分析揭示了侵袭性前列腺癌的生物学特征。","authors":"Vilde E Skingen, Unn Beate Salberg, Tord Hompland, Christina S Fjeldbo, Hanna Helgeland, Kari-Anne M Frikstad, Harald B Ragnum, Ljiljana Vlatkovic, Knut Håkon Hole, Therese Seierstad, Heidi Lyng","doi":"10.1002/path.6344","DOIUrl":null,"url":null,"abstract":"<p>Mechanisms regulating the gene expression program at different hypoxia severity levels in patient tumors are not understood. We aimed to determine microRNA (miRNA) regulation of this program at defined hypoxia levels from moderate to severe in prostate cancer. Biopsies from 95 patients were used, where 83 patients received the hypoxia marker pimonidazole before prostatectomy. Forty hypoxia levels were extracted from pimonidazole-stained histological sections and correlated with miRNA and gene expression profiles determined by RNA sequencing and Illumina bead arrays. This identified miRNAs associated with moderate (<i>n</i> = 7) and severe (<i>n</i> = 28) hypoxia and predicted their target genes. The scores of miRNAs or target genes showed prognostic significance, as validated in an external cohort of 417 patients. The target genes showed enrichment of gene sets for cell proliferation and MYC activation at all hypoxia levels and PTEN inactivation at severe hypoxia. This was confirmed by RT-qPCR for <i>MYC</i> and <i>PTEN</i>, by Ki67 immunohistochemistry, and by gene set analysis in an external cohort. To assess whether miRNA regulation occurred within the predicted hypoxic regions, a method to quantify co-localization of multiple histopathology parameters at defined hypoxia levels was applied. A high Ki67 proliferation index co-localized significantly with hypoxia at all levels. The co-localization index was strongly associated with poor prognosis. Absence of PTEN staining co-localized significantly with severe hypoxia. The scores for miRNAs correlated with the co-localization index for Ki67 staining and hypoxia, consistent with miRNA regulation within the overlapping regions. This was confirmed by showing miR-210-3p expression within severe hypoxia by <i>in situ</i> hybridization. Cell line experiments (22Rv1, PC3) were conducted to determine whether miRNAs and target genes were regulated directly by hypoxia. Most of them were hypoxia-unresponsive, and probably regulated by other mechanisms such as MYC activation. In conclusion, in aggressive, hypoxic prostate tumors, cancer cells exhibit different proliferative gene expression programs that is regulated by miRNAs and depend on whether the cells reside in moderate or severe hypoxic regions. © 2024 The Author(s). <i>The Journal of Pathology</i> published by John Wiley & Sons Ltd on behalf of The Pathological Society of Great Britain and Ireland.</p>","PeriodicalId":232,"journal":{"name":"The Journal of Pathology","volume":"264 3","pages":"270-283"},"PeriodicalIF":5.6000,"publicationDate":"2024-09-27","publicationTypes":"Journal Article","fieldsOfStudy":null,"isOpenAccess":false,"openAccessPdf":"https://onlinelibrary.wiley.com/doi/epdf/10.1002/path.6344","citationCount":"0","resultStr":"{\"title\":\"Spatial analysis of microRNA regulation at defined tumor hypoxia levels reveals biological traits of aggressive prostate cancer\",\"authors\":\"Vilde E Skingen, Unn Beate Salberg, Tord Hompland, Christina S Fjeldbo, Hanna Helgeland, Kari-Anne M Frikstad, Harald B Ragnum, Ljiljana Vlatkovic, Knut Håkon Hole, Therese Seierstad, Heidi Lyng\",\"doi\":\"10.1002/path.6344\",\"DOIUrl\":null,\"url\":null,\"abstract\":\"<p>Mechanisms regulating the gene expression program at different hypoxia severity levels in patient tumors are not understood. We aimed to determine microRNA (miRNA) regulation of this program at defined hypoxia levels from moderate to severe in prostate cancer. Biopsies from 95 patients were used, where 83 patients received the hypoxia marker pimonidazole before prostatectomy. Forty hypoxia levels were extracted from pimonidazole-stained histological sections and correlated with miRNA and gene expression profiles determined by RNA sequencing and Illumina bead arrays. This identified miRNAs associated with moderate (<i>n</i> = 7) and severe (<i>n</i> = 28) hypoxia and predicted their target genes. The scores of miRNAs or target genes showed prognostic significance, as validated in an external cohort of 417 patients. The target genes showed enrichment of gene sets for cell proliferation and MYC activation at all hypoxia levels and PTEN inactivation at severe hypoxia. This was confirmed by RT-qPCR for <i>MYC</i> and <i>PTEN</i>, by Ki67 immunohistochemistry, and by gene set analysis in an external cohort. To assess whether miRNA regulation occurred within the predicted hypoxic regions, a method to quantify co-localization of multiple histopathology parameters at defined hypoxia levels was applied. A high Ki67 proliferation index co-localized significantly with hypoxia at all levels. The co-localization index was strongly associated with poor prognosis. Absence of PTEN staining co-localized significantly with severe hypoxia. The scores for miRNAs correlated with the co-localization index for Ki67 staining and hypoxia, consistent with miRNA regulation within the overlapping regions. This was confirmed by showing miR-210-3p expression within severe hypoxia by <i>in situ</i> hybridization. Cell line experiments (22Rv1, PC3) were conducted to determine whether miRNAs and target genes were regulated directly by hypoxia. Most of them were hypoxia-unresponsive, and probably regulated by other mechanisms such as MYC activation. In conclusion, in aggressive, hypoxic prostate tumors, cancer cells exhibit different proliferative gene expression programs that is regulated by miRNAs and depend on whether the cells reside in moderate or severe hypoxic regions. © 2024 The Author(s). <i>The Journal of Pathology</i> published by John Wiley & Sons Ltd on behalf of The Pathological Society of Great Britain and Ireland.</p>\",\"PeriodicalId\":232,\"journal\":{\"name\":\"The Journal of Pathology\",\"volume\":\"264 3\",\"pages\":\"270-283\"},\"PeriodicalIF\":5.6000,\"publicationDate\":\"2024-09-27\",\"publicationTypes\":\"Journal Article\",\"fieldsOfStudy\":null,\"isOpenAccess\":false,\"openAccessPdf\":\"https://onlinelibrary.wiley.com/doi/epdf/10.1002/path.6344\",\"citationCount\":\"0\",\"resultStr\":null,\"platform\":\"Semanticscholar\",\"paperid\":null,\"PeriodicalName\":\"The Journal of Pathology\",\"FirstCategoryId\":\"3\",\"ListUrlMain\":\"https://onlinelibrary.wiley.com/doi/10.1002/path.6344\",\"RegionNum\":2,\"RegionCategory\":\"医学\",\"ArticlePicture\":[],\"TitleCN\":null,\"AbstractTextCN\":null,\"PMCID\":null,\"EPubDate\":\"\",\"PubModel\":\"\",\"JCR\":\"Q1\",\"JCRName\":\"ONCOLOGY\",\"Score\":null,\"Total\":0}","platform":"Semanticscholar","paperid":null,"PeriodicalName":"The Journal of Pathology","FirstCategoryId":"3","ListUrlMain":"https://onlinelibrary.wiley.com/doi/10.1002/path.6344","RegionNum":2,"RegionCategory":"医学","ArticlePicture":[],"TitleCN":null,"AbstractTextCN":null,"PMCID":null,"EPubDate":"","PubModel":"","JCR":"Q1","JCRName":"ONCOLOGY","Score":null,"Total":0}
引用次数: 0
引用
批量引用

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


